Abstract
Ataxia telangiectasia (AT) is an autosomal recessive disorder characterized by growth retardation, cerebellar ataxia, oculocutaneous telangiectasias, and a high incidence of lymphomas and leukemias. In addition, AT patients are sensitive to ionizing radiation. Atm-deficient mice recapitulate most of the AT phenotype. p21cip1/waf1 (p21 hereafter), an inhibitor of cyclin-dependent kinases, has been implicated in cellular senescence and response to γ-radiation-induced DNA damage. To study the role of p21 in ATM-mediated signal transduction pathways, we examined the combined effect of the genetic loss of atm and p21 on growth control, radiation sensitivity, and tumorigenesis. As might have been expected, our data provide evidence that p21 modifies the in vitro senescent response seen in AT fibroblasts. Further, it is a downstream effector of ATM-mediated growth control. In addition, however, we find that loss of p21 in the context of an atm-deficient mouse leads to a delay in thymic lymphomagenesis and an increase in acute radiation sensitivity in vivo (the latter principally because of effects on the gut epithelium). Modification of these two crucial aspects of the ATM phenotype can be related to an apparent increase in spontaneous apoptosis seen in tumor cells and in the irradiated intestinal epithelium of mice doubly null for atm and p21. Thus, loss of p21 seems to contribute to tumor suppression by a mechanism that operates via a sensitized apoptotic response. These results have implications for cancer therapy in general and AT patients in particular.
Ataxia telangiectasia (AT) is an autosomal recessive multiorgan disorder that is characterized by progressive cerebellar degeneration, immune deficiencies, growth retardation, premature aging, gonadal abnormalities, and sterility. In addition, AT patients are sensitive to ionizing radiation and manifest an increased incidence of lymphoid tumors (for review, see refs. 1–3). Telangiectasia (dilated blood vessels) appear later in the eyes and on the exposed skin of AT patients. This syndrome also shows multiple cellular defects, which include cellular senescence, cytoskeletal abnormalities, chromosomal instability, hypersensitivity to ionizing radiation, and defective cell cycle regulation response. ATM, the gene mutated in AT patients, has been identified through positional cloning (4). The 350-kDa ATM protein is similar to a family of proteins containing a highly conserved carboxy-terminal phospatidylinositol 3 kinase domain. Members of this family are involved in cell cycle regulation, telomere length monitoring, meiotic recombination, and DNA repair (for review, see refs. 1–3). However, despite its homology to lipid kinases, the ATM protein has been shown to possess only a serine-threonine phosphoprotein kinase activity, which is expressed ubiquitously in most tissues examined (13–16).
Mouse models for AT have been created (5–8) that recapitulate many aspects of the human AT phenotype. Atm mutant mice display pleiotropic defects, including growth retardation, gonadal atrophy, B and T cell deficiencies, and lymphoblastic lymphomas. Murine embryonic fibroblasts (MEFs) derived from atm null mice show chromosomal instability, require high serum for growth, senesce early in culture, are sensitive to ionizing radiation, and display defective G1/S cell-cycle checkpoint controls in response to DNA damage (5–8).
The induction of p53 is impaired in AT cells, indicating p53 acts downstream in the ATM signal transduction pathway (1–3). p53 encodes a transcription factor that is activated in response to DNA damage. One of the target genes that is regulated by p53 is the cyclin-dependent kinase inhibitor, p21. It is induced by DNA damage in a p53-dependent manner and arrests cell cycle in G1 when overexpressed (9, 10, 17). p21 induction also has been observed in cell lines undergoing differentiation or senescence in vitro (10, 17). p53 and p21 have been implicated in G1/S checkpoint control in response to ionizing radiation (11, 12, 17). However, the role of p53 and p21 in G2/M checkpoint control remains to be established. In addition, p21 is shown to be regulated in a p53-independent manner. For example, high levels of p21 expression are detected in intestine, lung, thymus, and testis in p53 null mice (18). Induction of p21 under oxidative stress also operates through a p53-independent pathway (19, 20).
Chronically high levels of p21 have been observed in lymphoblasts derived from AT patients and in atm-deficient mouse embryonic fibroblasts (8, 21, 22). These results suggest that p21 may mediate cellular senescence and lymphomagenesis in atm-deficient cells. In this report, we examine the effects of loss of p21 on cellular senescence, sensitivity to ionizing radiation, and lymphomagenesis in atm null mice and cell lines.
MATERIALS AND METHODS
Cell Culture.
All cells were derived from appropriate matings of mice carrying p21 and atm null alleles (6, 11). To produce MEFs of different genotypic combinations, compound heterozygotes (atm/+, p21/+) were mated, and timed pregnant females were sacrificed at 14.5–16.5 days postcoitum. Internal organs and heads of embryos were removed and used for Southern blot analysis. Embryonic carcasses were rinsed in iced PBS and minced. The embryonic fragments then were digested in trypsin and DNase1. The dissociated cells were then washed in PBS and plated in 10% fetal calf serum in DMEM.
Southern and Western Blot Analyses.
Genomic DNA was extracted from embryonic heads, MEFs, and tails and then digested in proteinase K and ethanol-precipitated. Genomic DNA then was digested with EcoRI and separated on a 1% agarose gel. Genomic DNA was blotted onto nylon membranes and hybridized to atm and p21 probes (6, 11). To analyze p21 expression, 50,000 MEF cells of each different genotype were collected and extracted in RIPA buffer (50 mM Tris⋅HCl. pH 7.4/1% Nonidet P-40/0.25% Na-deoxycholate/150 mM NaCl/1 mM EDTA/1 mM Na3VO4/1 mM NaF/1 μg/ml of aprotinin, leupeptin, and pepstatin). Cell suspensions were collected by centrifugation to remove cellular debris. Protein-containing extracts were mixed with equal volumes of 2× SDS buffer and loaded on a 15% SDS-polyacrylmide gel. After electrophoresis, the gel was semi-dry-transferred to a poly(vinylidene difluoride) membrane and blotted with p21 polyclonal antibody (Oncogene Science). Signal was detected with ECL chemiluminescence (Amersham). The membrane was stripped and reprobed with β-actin antibody (Sigma) under similar conditions.
Cell Proliferation and Saturation Density Assays.
MEFs (2 × 104) were triplicate-plated in a series of 35-mm dishes in DMEM containing 10% fetal calf serum. The cultures were maintained for up to 10 days. MEFs were trypsinized and counted in a hemocytometer, and new medium was added to the rest of the plates each day. Passage 4 MEFs were used.
Cell Cycle Analysis.
Asynchronized passage 2–3 MEFs in suspension medium were irradiated from a 137Cs source (Astrophysics Research, Long Beach, CA at 99 KV, 5 mA) at a dose rate of 2 Gy/min, replated in 10% fetal calf serum DMEM for 14 hr, and then pulsed with 10 μM 5-bromodeoxyuridine (BrdUrd) for 4 hr. Cells then were fixed in 70% ethanol. For G0-synchronization, asynchronous cells at 70% confluency were serum-starved for 96 hr in DMEM containing 0.5% fetal calf serum. Cells then were released into growth media for 24 hr with 65 μM BrdUrd. Subsequent fixation of cells was as above. Fixed cells were washed in PBS and treated with 0.1 N HCl containing 0.7% Triton X-100 to denature DNA. Cells then were incubated with anti-BrdUrd antibody (Becton–Dickson) and counterstained with propidium iodide containing RNase A. Replicative DNA synthesis and DNA content were analyzed by FACScan by using CellQuest software. DNA content also was obtained by analyzing data in Modfit LT software.
Radiation Sensitivity.
Mice of different genotypes at age 35–40 days were irradiated with 8 Gy or 10 Gy at a dose of 2.03 Gy/min with a 137Cs source (Mark 1 Irradiator, J. L. Shepherd & Sons, San Bernardino, CA). Mice subsequently were housed together and examined daily for clinical symptoms.
Histology.
Mouse tissues or tumors were dissected, fixed in Optimal Fix (American Histology Regent, Lodi, CA), dehydrated, embedded in paraffin, sectioned to 7 μm, and stained with haematoxylin and eosin. Tissue images were obtained with a Sony digital camera and analyzed by a pathologist.
RESULTS
Loss of p21 Rescues Cellular Senescence but Not the G1/S Checkpoint Defect in atm-Deficient Fibroblasts.
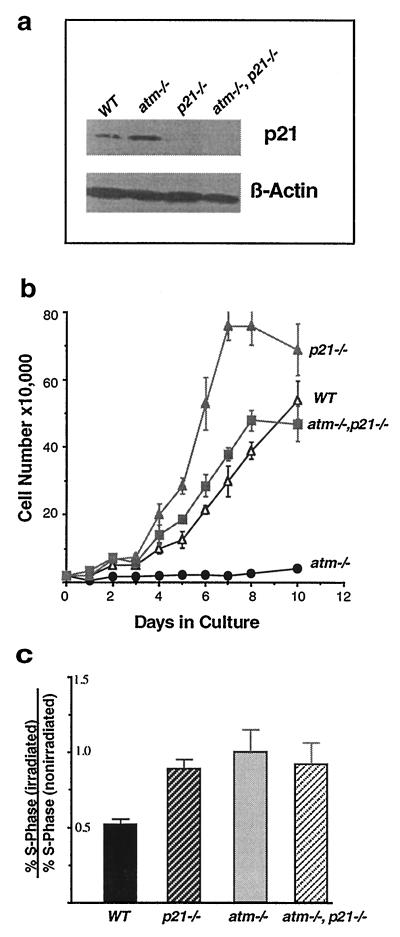
Atm-deficient fibroblasts senesce early in culture and show increased levels of p21 (7, 8, 22). To study the role of p21 in ATM-mediated signal transduction pathways and in aspects of the atm phenotype, we generated mice and MEFs that were doubly null for atm and p21. A total of 58 primary MEF cell lines were derived from doubly heterozygotic matings. Among the anticipated genotypic combinations, four MEF cell lines deficient for both ATM and p21 were identified by Southern blot analysis. As shown in Fig. 1a, p21 protein levels are higher in atm null MEFs than in wild-type MEFs, whereas—as expected—p21 protein is absent from p21 null and atm/p21 double-null MEFs. The growth properties of these MEFs were examined in early passage cells (passage 4) (Fig. 1b). Consistent with earlier observations, atm null MEFs appear senescent and grow poorly. By contrast, p21 null MEFs grow rapidly and are contact-inhibited only at high density. As shown in Fig. 1b, the loss of p21 in an atm null background dramatically alleviates the growth suppressive phenotype of the atm null cells. The growth rate of atm/p21 double-null cells is comparable to that of wild-type cells and saturates at a similar density. In contrast to the early senescent behavior of the atm null cells, atm/p21 double-null cells do not growth arrest and can be carried beyond passage 20 (data not shown). Thus, loss of p21 in atm null MEF alleviates growth arrest, suggesting that p21 plays a role in the senescent behavior observed in AT MEFs, and that p21 acts downstream in growth control regulated by ATM.
Figure 1.
Cell proliferation and cell cycle analysis. (a) Western blot analysis of cell lines used in this study. Lane 1, wild-type MEF cells. Lane 2, atm null MEF cells. Lane 3, p21 null MEF cells. Lane 4, atm/p21 double-null MEF cells. Increased expression of p21 is seen in atm null as compared with wild-type cells. p21 expression is not seen in both p21 null and atm/21 double-null cells. A β-actin control for equal loading also is shown. (b) Saturation densities and growth properties of the MEFs. MEFs (2 × 104) at passage 4 were plated in triplicate on 35-mm culture dishes, and medium was changed every day. The cell number at each time point represents the mean value of triplicates with an error bar showing standard deviation. The genotype of each cell line is indicated. atm/p21 double-null cells grow at a similar rate to wild type but slower than p21 null cells and also arrest at a lower saturation density. (c) G1/S checkpoint defect in atm/p21 double-null MEFs in response to ionizing radiation. Unsynchronized atm, atm/p21, p21, and wild-type MEFs were irradiated at 0 and 10 Gy. Fourteen hours after irradiation, cells were pulsed with 10 μM of BrdUrd for 4 hr. Cells were fixed and stained with anti-BrdUrd antibody and propidium iodide and analyzed by FACS for S-phase and DNA contents. Three independent experiments were performed at the indicated dosage. The ratios of the percentage of irradiated S-phase cells versus the percentage of nonirradiated S-phase cells are presented with error bars showing standard deviations. Solid bar, wild type; dark hatched bar, p21/p21; light solid bar, atm/atm; light hatched bar, atm/p21.
Because both atm and p21 single-null cells are defective in their response to DNA damage (8, 11, 12, 23), it is tempting to suspect that p21 exerts its effects on in vitro MEF growth via its role in the DNA radiation damage checkpoint. To understand the epistatic relationship between ATM and p21 in DNA-damage response and cell cycle regulation, asynchronously growing early passage cells of atm null, p21 null, atm/p21 double-null, and wild-type genotypes were irradiated at 10 Gy and analyzed for cell cycle stage by FACS 18 hr after irradiation (Fig. 1c). Wild-type cells demonstrated a 50% reduction in S-phase cells, whereas atm, p21, and atm/p21 null cells did not show any significant reduction in S-phase cells after irradiation, indicating that the G1/S checkpoint is defective in these mutant cell lines (Fig. 1c). This result suggests that ATM and p21 invoke G1/S checkpoint control on the same pathway in response to DNA damage induced by ionizing radiation. One also might imagine that radiation-independent DNA damage incurred as a result of the atm deficiency (which leads to an increase in p21; Fig. 1a) represses cell growth via p21. Thus, loss of p21 relieves cell cycle checkpoint(s) control.
Loss of p21 Leads to Increased Sensitivity to Ionizing Radiation in atm-Deficient Mice.
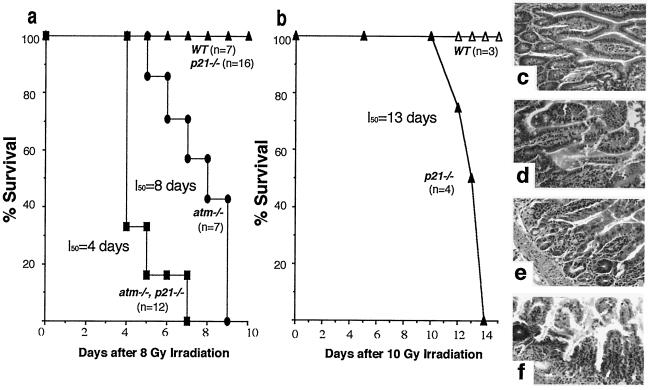
AT mice, like AT patients, are sensitive to ionizing radiation and exhibit acute gut cytoxicity (4). In normal irradiated mice, intestinal epithelial cells undergo apoptosis in a p53-dependent manner (24, 25). Interestingly, however, p21 is expressed at high levels in intestinal tissues in a p53-independent manner (18). It was therefore of interest to study the interaction of atm and p21 in radiation-mediated gut toxicity. To do this study, 5-week-old atm/p21 double-null mice were irradiated at 8 Gy and compared with atm null, p21 null, and wild-type mice of the same age. Loss of p21 severely exacerbated the acute radiation sensitivity of AT mice. As shown in Fig. 2a, 50% of the atm/p21 double-null mice survived until day 4 postirradiation, whereas a similar fraction of the single-null AT mice survived until day 8. The difference observed is statistically significant when using the Wilcoxon two-sample test assuming nonparametric data and a two-tail P value (P < 0.002). All of the wild-type and p21 null mice survived beyond day 12. Indeed, 16 p21 null mice were followed and only one of these mice died at 14 days postirradiation. All 20 atm heterozygotes in the p21 null background survived beyond 30 days (data not shown).
Figure 2.
Acute radiation hypersensitivity in atm/p21 double-null mice. Survival curves are shown of mice irradiated at (a) 8 Gy and (b) 10 Gy. Statistical analysis was performed by using Wilcoxon two-sample test assuming nonparametric data and a two-tailed P value (P < 0.002). Genotypes of mice are indicated. Data in a are combined from two separate sets of experiments. Hematoxylin and eosin-stained sections of the small intestine taken at day 3 after 8 Gy irradiation are shown in c-f at ×40 magnification. (c) Wild type. (d) p21/null. (e) atm/null. (f) atm/p21 double null. p21 null mice irradiated at 10 Gy show edematous degeneration of small intestine 3 days postirradiation (data not shown).
The accelerated kinetics of acute radiation toxicity observed in the atm/p21 double-null mice suggest that ATM and p21 normally cooperate in preventing radiation-induced apoptosis in the intestinal epithelium. To demonstrate that p21 plays a role in protecting intestinal epithelial cells from radiation-induced apoptosis, we irradiated a group of p21 null mice at 10 Gy and observed that 50% of p21 null mice succumbed by day 13, whereas all of the wild-type mice survived (Fig. 2b). In a separate experiment designed to study the morphologic manifestations of hypersensitivity to ionizing radiation, five mice each of wild-type, atm, p21, and atm/p21 single- and double-null genotypes were irradiated at 8 Gy, and sections of intestine taken at days 2, 3, and 4 were examined histologically. Consistent with the result presented in Fig. 2a, all atm/p21 double-null mice exhibited symptoms of lethargy and malaise as early as 2 days postirradiation, whereas atm, p21 single-null, and wild-type mice appeared to be normal (data not shown). This observation is supported by the increased edematous degeneration seen in atm/p21 double-null mouse intestinal epithelium and crypts (Fig. 2f) as compared with atm single null (Fig. 2e). In contrast, the intestines of p21 null mice (Fig. 2d) exhibit only minor signs of edematous degeneration of the normal architecture, whereas wild-type intestines (Fig. 2c) are entirely normal. This result suggests that ATM and p21 cooperate to prevent radiation-induced apoptosis.
Loss of p21 Delays the Onset of Lymphoma in atm-Deficient Mice.
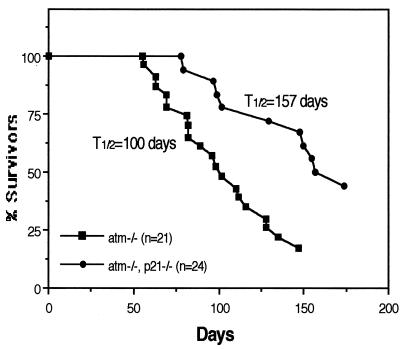
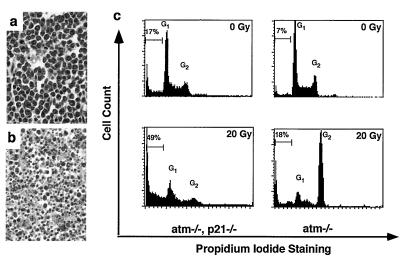
Early studies in p21-deficient mice suggest that p21 does not behave as a tumor suppressor in vivo, tumor incidence in these mice being no different than in wild type (11). Nevertheless, the finding of chronically higher levels of p21 in lymphoblasts derived from AT patients (21) suggested an interaction, likely indirect, between these genes. Accordingly, we wanted to assess the role of p21 deficiency on the highly increased incidence of lymphoma in AT mice. Mice of all possible atm/p21 genotypes were bred and observed for tumor formation (largely thymic lymphomas). Interestingly, loss of p21 led to a greater than 50-day delay in the onset of lymphomas in AT mice. As shown in Fig. 3, 50% of the atm null mice developed tumors and died by 100 days of age, whereas 50% of mice deficient in both ATM and p21 survived beyond 157 days. Surprisingly, loss of p21 had a suppressive effect on tumor formation. Pathologic examination of the thymomas themselves provides a strong hint as to the basis of the potential effect of p21 loss. Thymomas derived from atm/p21 double-null mice show high levels of apoptotic cells in the tumor (n = 4 mice), whereas apoptotic cells are not observed in tumor samples from atm null mice (n = 7 mice) (Fig. 4 a and b). The apoptotic cells in the tumors were confirmed by direct FACS analysis of tumor samples stained with annexin V and propidium iodide (data not shown).
Figure 3.
atm/p21 double-null mice show delayed tumorigenesis. Tumor-free mouse survival curves are shown. Genotypes and numbers of mice are indicated. Fifty percent of the atm null and atm/p21 double-null mice die before the ages of 100 and 157 days, respectively. Most of the atm and atm/p21 mice died of thymic lymphoma. None of the p21 null (n = 7) and wild-type (n = 16) mice became ill at the indicated times.
Figure 4.
atm/p21 double-null tumors exhibit increased apoptosis. (a and b) Hematoxylin and eosin staining of tumor section. ×40 magnification. (a) An atm thymic lymphoma shows uniform T lymphoblastic cells with no detectable signs of apoptosis. (b) An atm/p21 double-null thymic lymphoma shows extensive apoptosis. (c) DNA damage-induced defective G2/M checkpoint control in atm/p21 double-null thymomas leads to increased apoptosis in thymic tumor cells. Tumor cell lines were irradiated at 0 and 20 Gy. Cells were collected at 24 hr, fixed and stained with propidium iodide, and analyzed for DNA content and apoptotic cells. G1 and G2 peaks are indicated as well as percentage of apoptotic cells. Similar observations were seen at 5 and 10 Gy of irradiation and from independent cell lines (not shown).
Our studies suggest that a normal function of p21 may be to protect thymic tumor cells against apoptosis and that loss of p21 potentiates the apoptotic response induced by intrinsic or extrinsic means. To test this notion more directly, thy1 positive tumor cell lines were derived from the thymic lymphomas of both atm and atm/p21 null mice to test their propensity to undergo apoptosis spontaneously in vitro. Consistent with the in vivo finding, an atm/p21 double-null thymoma cell line showed increased spontaneous apoptosis in comparison to an atm thymoma cell line (17% vs. 7%, Fig. 4c). A dose- and time-dependent study of the radiation response of these cell lines also was performed. Both atm and atm/p21 thymoma cell lines showed defective G1 checkpoint control after irradiation at 20 Gy (5 Gy and 10 Gy data not shown) as analyzed by FACS (Fig. 4c). Interestingly, the atm cell lines exhibited a normal G2 arrest in a dose- and time-dependent manner, whereas atm/p21 cell lines lack this G2 arrest (Fig. 4c). The consequence of such a lack of G2 arrest is the dramatic increase in apoptosis observed at 24 hr postirradiation in the atm/p21 lymphoma cells (49% vs. 18% in atm lymphoma cells).
DISCUSSION
Chronically high levels of p21 expression have been observed in AT lymphoblasts and atm-deficient murine fibroblast cell lines (8, 21, 22). These results suggested that p21 might mediate cellular senescence, lymphomagenesis, and other features of the AT phenotype. To examine the role of p21 in ATM-mediated signal transduction pathways, we have used atm null mice and cell lines to analyze the effects of loss of p21 on the cardinal features of the AT phenotype. Not surprisingly, given the role p21 plays in cell cycle checkpoint control, we observed that loss of p21 could rescue cellular senescence in atm-deficient fibroblast growth in culture. Quite surprisingly, however, we observed that double-null mice exhibited an increased hypersensitivity to ionizing radiation and a striking delay of the onset of tumorigenesis.
Increased cellular levels of p21 are associated with terminal differentiation and cellular senescence (10). In addition, induced p21 expression can be seen under conditions of DNA damage and oxidative stress (19, 20, 26). Atm-deficient fibroblasts grown in culture show severe proliferative defects and senesce. These effects are correlated with an elevated level of p21 (8, 22). Here we have shown that genetic deletion of p21 in an atm-deficient background can rescue cells from senescence and overcome the growth arrest seen in atm null fibroblasts. This result indicates that p21 is a key regulator of cellular senescence and that p21 acts downstream in ATM-mediated growth regulation. Although loss of p21 rescues growth deficiency seen in atm null cell lines, it is important to point out that p21 null fibroblasts grow faster and saturate at a higher density than atm and p21 double-null cell lines (Fig. 1b). This result suggests that additional growth inhibitors may cooperate with p21 to mediate the growth suppression seen in atm null fibroblasts. Our data are consistent with results that showed that genetic deletion of p53 in the atm null background suppresses growth arrest in atm-deficient fibroblasts and suggested that this suppression might be because of loss of p21 expression (22). Because a similar growth rate was observed between p53 single-null and p53/atm double-null cell lines (22), it is possible that the expression of additional growth inhibitors besides p21 are suppressed by the loss of p53 in the atm null cell lines (22). In addition, our notion that p21 is a central mediator in vitro of cellular senescence is supported by the recent observation that genetic deletion of p21 bypasses cellular senescence in a human diploid fibroblast cell line (27).
Atm null fibroblasts show multiple defects in cell cycle checkpoints. Indeed, one of the hallmarks of the AT phenotype is radioresistant-DNA synthesis (1–3). p21 null fibroblasts also exhibit radioresistant DNA synthesis and defective G1/S checkpoint control (11, 12). The availability of mice and cells selectively deficient in both of these genes has allowed us to examine the epistatic relationship between atm and p21 in response to DNA damage and cell cycle regulation. Atm and p21 double-null fibroblasts show a G1/S checkpoint defect in response to ionizing radiation that is similar to that seen in atm or p21 single-null fibroblasts. Our data further indicate that the atm- and p21-mediated G1/S checkpoint control pathways are overlapping. It has been demonstrated that the DNA damage checkpoint pathways induced by UV are intact in AT cell lines (3), whereas p21 has been implicated in the regulation of UV-induced DNA damaged checkpoint pathway (26). Accordingly, we find that atm and p21 double-null fibroblasts show G1/S checkpoint defects that are seen in p21 null cells, but not in atm null cells (data not shown).
Atm null mice show growth retardation at weaning that persists through adult life (5–8). Atm and p21 double-null mice also show growth retardation with about a 30% reduction of body weight as compared with wild-type mice and p21 null mice (data not shown). Thus, loss of p21 in atm-deficient mice does not rescue the growth retardation phenotype. Loss of atm also leads to infertility in both male and female mice (5, 6, 8). It was shown that infertility is caused by defects in spermatogenesis and oogenesis. Histological examination of ovaries and testis from atm/p21 double-null reveal defects that are similar to those seen in atm null mice, with absence of mature spermatids and degeneration of spermatocytes in testis and missing primary oocytes and follicles in ovaries (data not shown). In addition, we do not observe any overt ataxia in atm and p21 double-null mice, although ataxia is a profound phenotype seen in AT patients.
Atm-deficient mice, like AT patients, are sensitive to ionizing radiation and exhibit acute gut cytoxicity (5). The earliest lethal in vivo effects of ionizing radiation are largely caused by toxic effects on the intestinal epithelium. In normal irradiated mice, intestinal epithelial cells undergo apoptosis in a p53-dependent manner (24, 25). Interestingly, atm/p53 double-null mice exhibit a level of gut toxicity that is similar to that of atm null mice (28), invoking a different cytotoxic pathway that is p53-independent. It was shown previously that high levels of p21 expression can be detected in intestinal tissue, with the expression being independent of p53 (18). Because radiation hypersensitivity is a serious and confounding feature of AT, it was important to assess the role of p21 in this response. Our results show that atm/p21 double-null mice are more sensitive to ionizing radiation than atm single-null mice (P < 0.002). This finding is supported further by histologic analysis of irradiated intestinal tissues in which progressively more severe intestinal degeneration is seen in atm/p21 double-null mice than in atm single-null mice. Our results suggest that ATM and p21 might cooperate to suppress radiation-induced apoptosis in the intestinal epithelium. It has been suggested that intestinal epithelial cells, in particular crypt cells, arrest cell cycle progression in the G2 phase in response to ionizing radiation (24). It is conceivable that ATM and p21 might be involved in the G2/M checkpoint control in the intestinal epithelial cells. Loss of atm or p21 in intestinal epithelial cells would abolish the G2/M checkpoint control and lead to increased apoptosis in response to ionizing radiation. This hypothesis might be the basis of the increased radiation sensitivity seen in atm/p21 double-null mice.
Recent studies have shown that loss of atm in conjunction with loss of p53 accelerates lymphomagenesis in double-null (as compared with single-null) mice (28). Mice genetically deficient in p21 (unlike p53-deficient mice) are not tumor prone, suggesting that the tumorigenic effect of loss of p53 might occur through loss of the p53-dependent apoptotic pathway. In addition, elevated levels of p21 are observed in AT lymphoma cell lines, suggesting an interaction between these genes during lymphomagenesis. Interestingly, we show here that loss of p21 in atm null mice delays the onset of tumor formation. Fifty percent of atm null mice develop thymic lymphomas by 100 days of age, whereas the onset of lymphomas is delayed—on average—more than 50 days in atm/p21 double-null mice. Furthermore, we show that the delayed tumorigenesis is accompanied by elevated levels of apoptosis seen in atm/p21 double-null thymomas. Such results suggest that the function of p21 in some malignant cells might be to block access to an apoptotic pathway. Tumor cell lines derived from either atm null mice or atm/p21 double-null mice were examined for the response to DNA damage induced by ionizing radiation. Consistent with the previous observation, atm null lymphoma cell lines show defective G1/S checkpoint control but arrest normally at G2. Interestingly, G2 arrest is defective or abolished in atm/p21 double-null lymphoma cell lines. The possible consequence of this loss of G2 arrest might be the dramatic increase in apoptotic cells we observe postirradiation (Fig. 4c). This result suggests that p21 might actively monitor G2/M checkpoint control in these lymphoma cells. Perhaps not surprisingly, it was shown that colon cancer cell lines deficient for p21 undergo apoptosis in response to DNA damage (29). Although a delayed apoptotic response is seen in p21-deficient colon cancer cells (29), the apoptotic response we observe in atm/p21-deficient lymphoma cell lines appears to occur earlier. It is thus interesting that in both cases, loss of p21 leads to a DNA damage-induced defect in the G2/M checkpoint in tumor cell lines (lymphoblastic cells and colon epithelial cells) with subsequent susceptibility to apoptosis. In addition, high levels of p21 protein are associated with chemoresistance in acute myelogenous leukemia (30). The findings that introduction of p21 antisense oligos induces apoptosis, whereas overexpression of p21 protects against apoptosis in vitro (31–33) are consistent with the results presented in this report. It is of interest to note that p57KIP2 (a member of the p21 cyclin-dependent kinase inhibitor family) null mice show marked increases in apoptosis in lens epithelial cells (34).
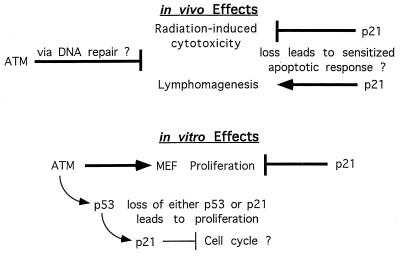
Our results demonstrate that p21 is an essential factor in ATM-mediated growth regulation and cellular senescence. In addition, we have demonstrated that p21 and ATM play distinctive and cooperative roles in checkpoint control and apoptosis in response to DNA damage. A summary of the effects we observe and the potential involvement of apoptotic and cell cycle checkpoint mechanisms are shown in Fig. 5. Loss of atm leads to radiation sensitivity and lymphomagenesis, possibly through defective DNA repair or replication. Loss of p21 in this ATM-deficient context stimulates acute radiation sensitivity (intestinal cytoxicity), but delays lymphomagenesis, both possibly by triggering an apoptotic response. Apparently by using a different pathway, p21 relieves the in vitro senescent response seen in MEFs, most likely via relief of the G1/S cell cycle checkpoint. Drugs that specifically inhibit p21 function in cancer cells (or the function of other proteins in its signal pathway), in combination with DNA damaging agents, could lead to more efficient tumor therapy. Our findings provide a framework for further studies relevant to cancer therapy in general and AT patients in particular.
Figure 5.
Summary of in vivo and in vitro effects of p21 in the context of AT. Arrows indicate inducing effects; blunted arrows indicate inhibitory effects. The indicated effects of p21 are observed in the context of an atm null mouse or cell. Statements or pathways followed by a question mark indicate a proposed mechanism. The summary diagram is described in the text.
Acknowledgments
Special thanks go to R. Cardiff for analysis of mouse pathological samples and for helpful suggestions, to B. Leader for help with statistical analysis, to R. Van Etten for instructions in the use of the irradiator, to C. H. Westphal, N. Chester, and Y. Ishida for helpful discussions and comments on the manuscript. Y.A.W. is a Postdoctoral Associate and P.L. is a Senior Investigator of the Howard Hughes Medical Institute. A.E. is an Alon Fellow and incumbent of the Adolfo and Evelyn Blum Career Development Chair in Cancer Research at The Weizmann Institute.
ABBREVIATIONS
- AT
ataxia telangiectasia
- MEF
murine embryonic fibroblast
- BrdUrd
5-bromodeoxyuridine
References
- 1.Meyn M S. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 2.Jorgenson T J, Shiloh Y. Int J Rad Biol. 1996;69:527–537. doi: 10.1080/095530096145535. [DOI] [PubMed] [Google Scholar]
- 3.Lavin M F, Shiloh Y. Annu Rev Immunol. 1996;54:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, et al. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 5.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 6.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Baltimore D. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- 9.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 10.Harper J W, Elledge S J. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 11.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 12.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Lee E. J Biol Chem. 1996;271:33693–33697. doi: 10.1074/jbc.271.52.33693. [DOI] [PubMed] [Google Scholar]
- 14.Keegan K S, Holtzman D A, Plug A W, Christenson E R, Brainerd E, Flaggs G, Bentley N J, Taylor E M, Meyn M S, Moss S B, Carr A M, Ashley T, Hoekstra M F. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- 15.Jung M, Kondratyev A, Lee S A, Dimtchev A, Dritschilo A. Cancer Res. 1997;57:24–27. [PubMed] [Google Scholar]
- 16.Baskaran R, Wood L D, Whitaker L L, Canman C E, Morgan C E, Xu Y, Barlow C, Baltimore D, Wynshaw-Boris A, Kastan M B, Wang J Y. Nature (London) 1997;387:516–519. doi: 10.1038/387516a0. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 18.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 19.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor P M, Anderson C W, Appella E. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 20.Qiu X, Forman H J, Schonthal A H, Cadenas E. J Biol Chem. 1996;271:31915–31921. doi: 10.1074/jbc.271.50.31915. [DOI] [PubMed] [Google Scholar]
- 21.Beamish H, Williams R, Chen P, Lavin M F. J Biol Chem. 1996;271:20486–20493. doi: 10.1074/jbc.271.34.20486. [DOI] [PubMed] [Google Scholar]
- 22.Westphal C H, Schmaltz C, Rowan S, Elson A, Fisher D E, Leder P. Cancer Res. 1997;57:1664–1667. [PubMed] [Google Scholar]
- 23.Waldman T, Kinzler K W, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 24.Potten C S, Owen G, Roberts S A. Int J Rad Biol. 1990;57:185–199. doi: 10.1080/09553009014550431. [DOI] [PubMed] [Google Scholar]
- 25.Clarke A R, Gledhill S, Hooper M L, Bird C C, Wyllie A H. Oncogene. 1994;9:1767–1773. [PubMed] [Google Scholar]
- 26.Elledge S J, Harper J W. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 27.Brown J P, Wei W, Sedivy J M. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 28.Westphal C H, Schmaltz C, Rowan S, Elson A, Fisher D E, Leder P. Nat Genet. 1997;16:397–401. doi: 10.1038/ng0897-397. [DOI] [PubMed] [Google Scholar]
- 29.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Kornblau S M, Kobayashi T, Gambel A, Claston D, Deisseroth A B. Clin Cancer Res. 1995;1:1051–1057. [PubMed] [Google Scholar]
- 31.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorospe M, Wang X, Guyton K Z, Holbrook N J. Mol Cell Biol. 1996;16:6654–6660. doi: 10.1128/mcb.16.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorospe M, Cirilli C, Wang X, Seth P, Capogrossi C, Holbrook N J. Oncogene. 1997;14:929–935. doi: 10.1038/sj.onc.1200897. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Liegeois N J, Wong C, Finegold M, Hou H, Thompson J C, Silverman A, Harper J W, DePinho R A, Elledge S J. Nature (London) 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]