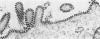Abstract
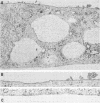
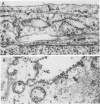
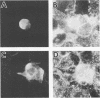
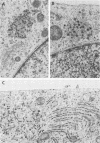
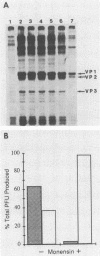
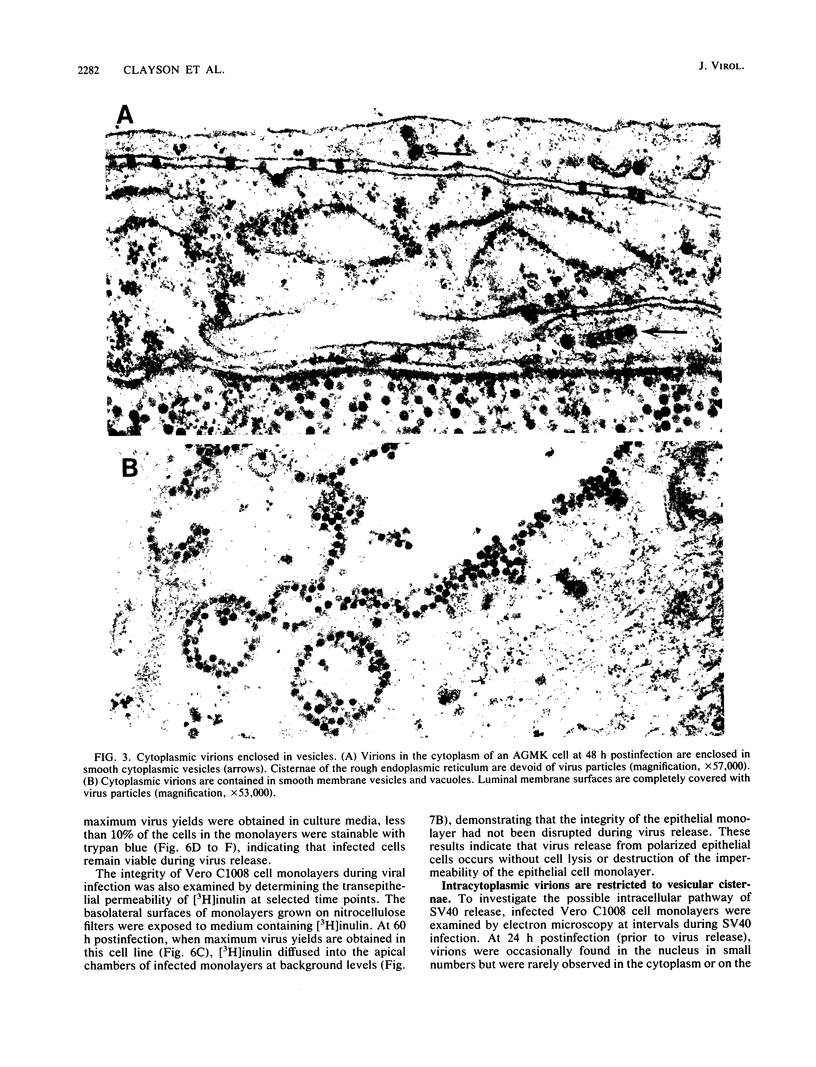
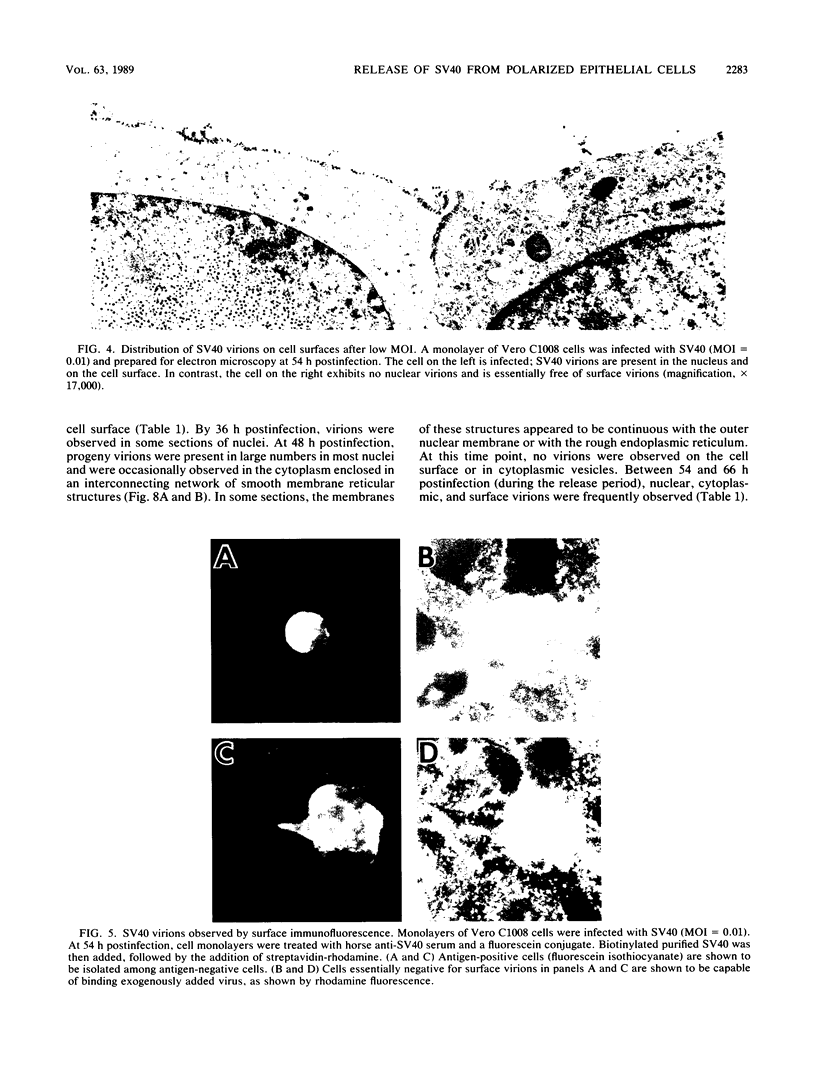
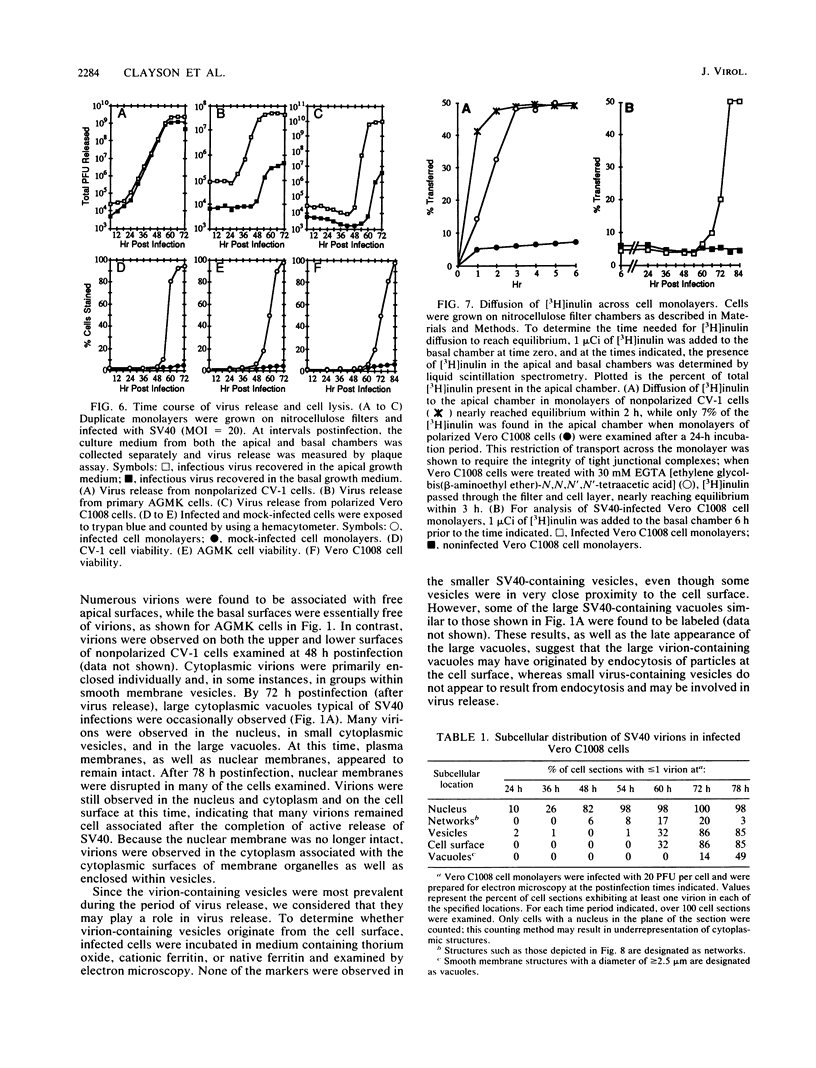
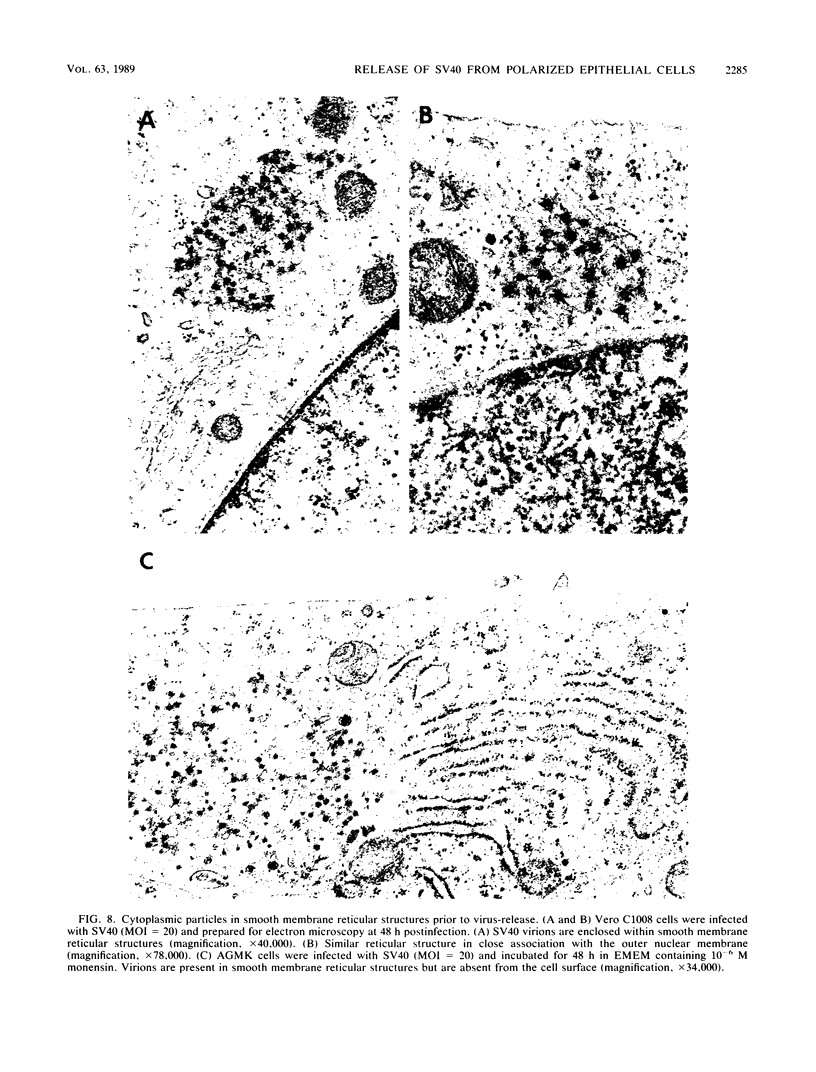
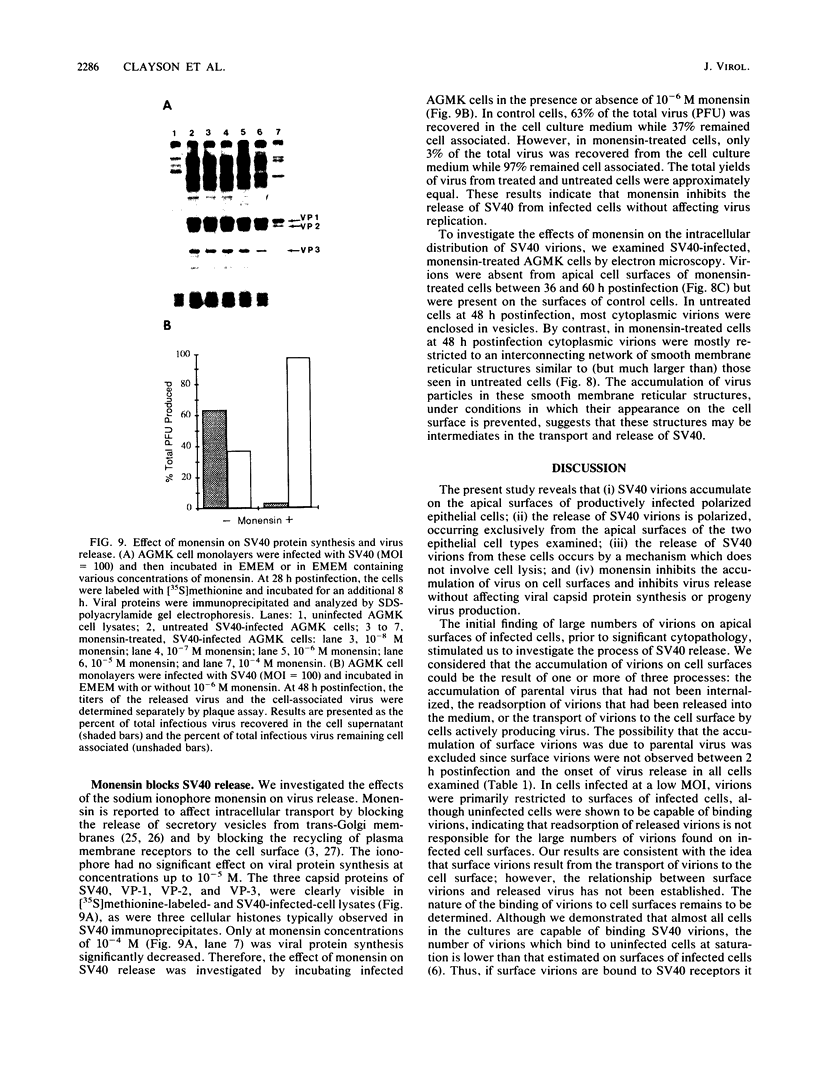
We have investigated the process of release of simian virus 40 (SV40) virions from several monkey kidney cell lines. High levels of virus release were observed prior to any significantly cytopathic effects in all cell lines examined, indicating that SV40 utilizes a mechanism for escape from the host cell which does not involve cell lysis. We demonstrate that SV40 release was polarized in two epithelial cell types (Vero C1008 and primary African green monkey kidney cells) grown on permeable supports; release of virus occurs almost exclusively at apical surfaces. In contrast, equivalent amounts of SV40 virions were recovered from apical and basal culture fluids of nonpolarized CV-1 cells. SV40 virions were observed in large numbers on apical surfaces of epithelial cells and in cytoplasmic smooth membrane vesicles. The sodium ionophore monensin, an inhibitor of vesicular transport, was found to inhibit SV40 release without altering viral protein synthesis or infectious virus production.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Induced latent infection of monkeys with vacuolating SV-40 Papova virus. Virus in kidneys and urine. Proc Soc Exp Biol Med. 1962 Nov;111:367–372. doi: 10.3181/00379727-111-27794. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. H., Scott F. W. Feline panleukopenia. II. The relationship of intestinal mucosal cell proliferation rates to viral infection and development of lesions. Vet Pathol. 1977 Mar;14(2):173–181. doi: 10.1177/030098587701400209. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B. J., Carmichael L. E., Appel M. J., Greisen H. Canine viral enteritis. II. Morphologic lesions in naturally occurring parvovirus infection. Cornell Vet. 1979 Apr;69(3):134–144. [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr Rhinoviruses. Yale J Biol Med. 1975 Mar;48(1):17–45. [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Jones L. V., Compans R. W., Davis A. R., Bos T. J., Nayak D. P. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol Cell Biol. 1985 Sep;5(9):2181–2189. doi: 10.1128/mcb.5.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MEYER H. M., Jr, HOPPS H. E., ROGERS N. G., BROOKS B. E., BERNHEIM B. C., JONES W. P., NISALAK A., DOUGLAS R. D. Studies on simian virus 40. J Immunol. 1962 Jun;88:796–806. [PubMed] [Google Scholar]
- Maul G. G. Fibrils attached to the nuclear pore prevent egress of SV40 particles from the infected nucleus. J Cell Biol. 1976 Sep;70(3):714–719. doi: 10.1083/jcb.70.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C., Ouellette J. Cell killing by simian virus 40: variation in the pattern of lysosomal enzyme release, cellular enzyme release, and cell death during productive infection of normal and simian virus 40-transformed simian cell lines. J Virol. 1976 Apr;18(1):48–57. doi: 10.1128/jvi.18.1.48-57.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro L. S., Rose H. M., Morgan C., Hsu K. C. Electron microscopic study of the development of simian virus 40 by use of ferritin-labeled antibodies. J Virol. 1967 Apr;1(2):384–399. doi: 10.1128/jvi.1.2.384-399.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Sharma S., Rodgers L., Brandsma J., Gething M. J., Sambrook J. SV40 T antigen and the exocytotic pathway. EMBO J. 1985 Jun;4(6):1479–1489. doi: 10.1002/j.1460-2075.1985.tb03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Balachandran N., Alonso-Caplen F. V., Compans R. W. Expression of herpes simplex virus glycoproteins in polarized epithelial cells. J Virol. 1986 May;58(2):689–693. doi: 10.1128/jvi.58.2.689-693.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Willemsen R., van Kerkhof P., Slot J. W., Geuze H. J., Lodish H. F. Vesicular stomatitis virus glycoprotein, albumin, and transferrin are transported to the cell surface via the same Golgi vesicles. J Cell Biol. 1983 Dec;97(6):1815–1822. doi: 10.1083/jcb.97.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]