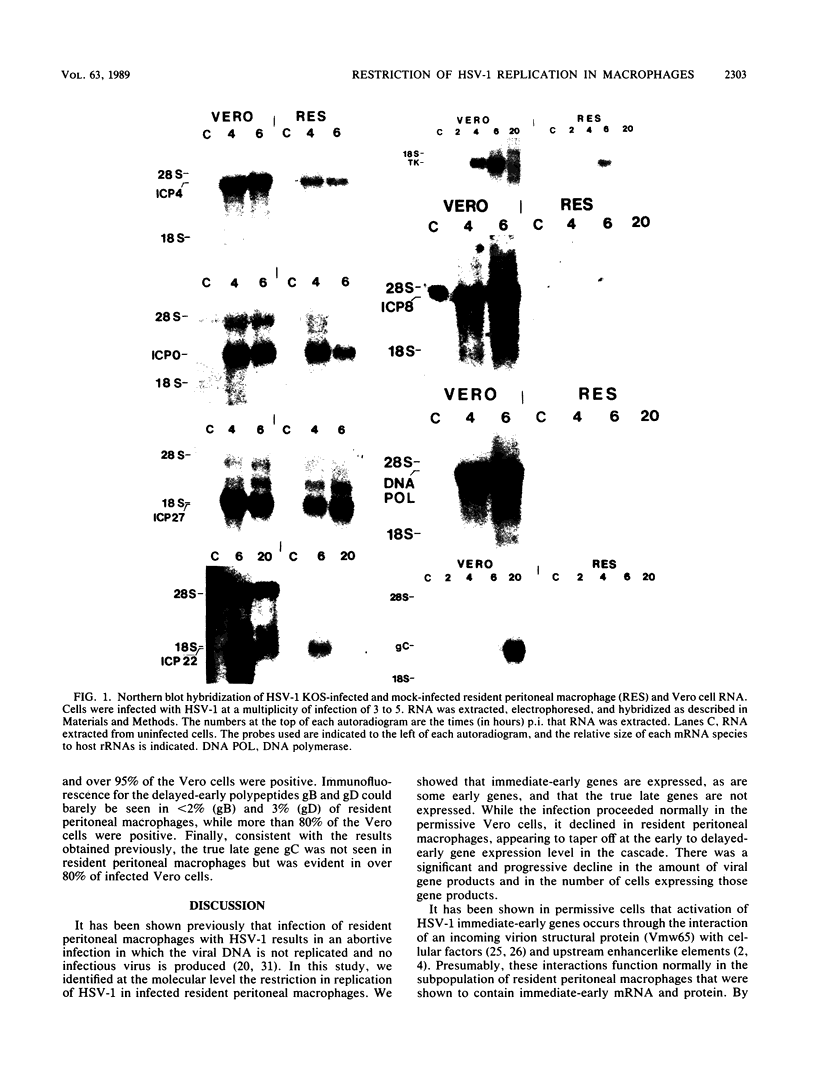
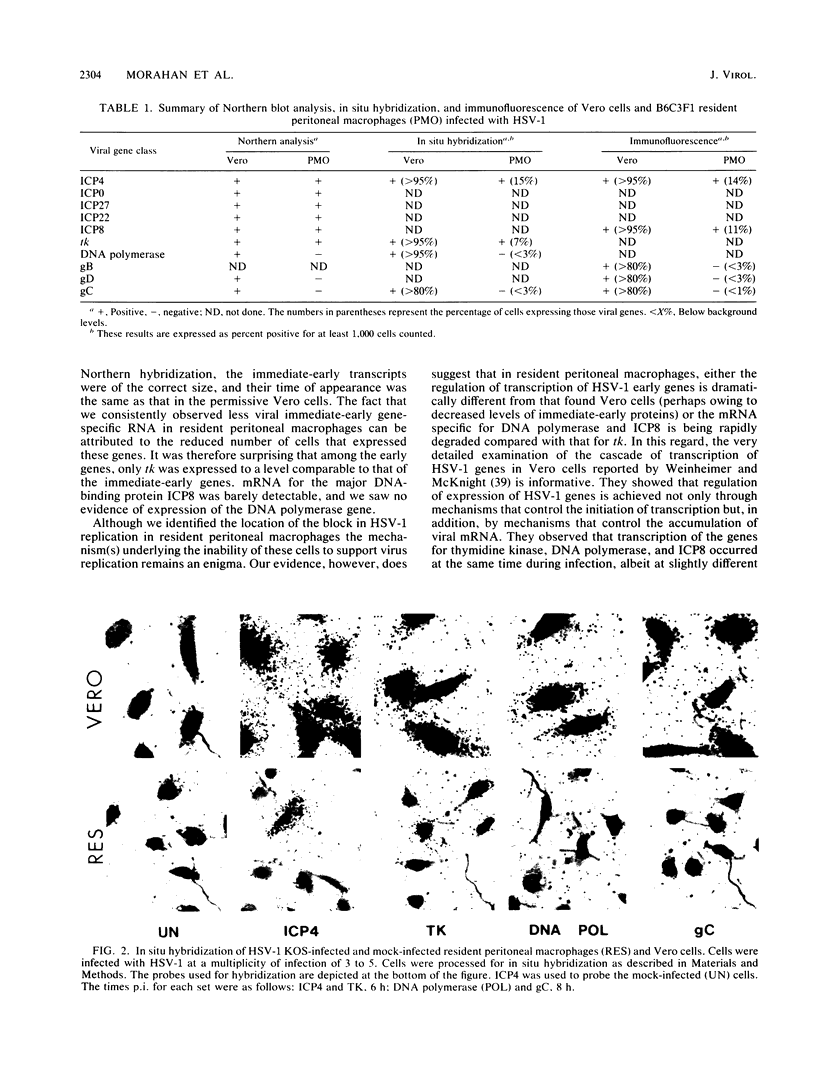
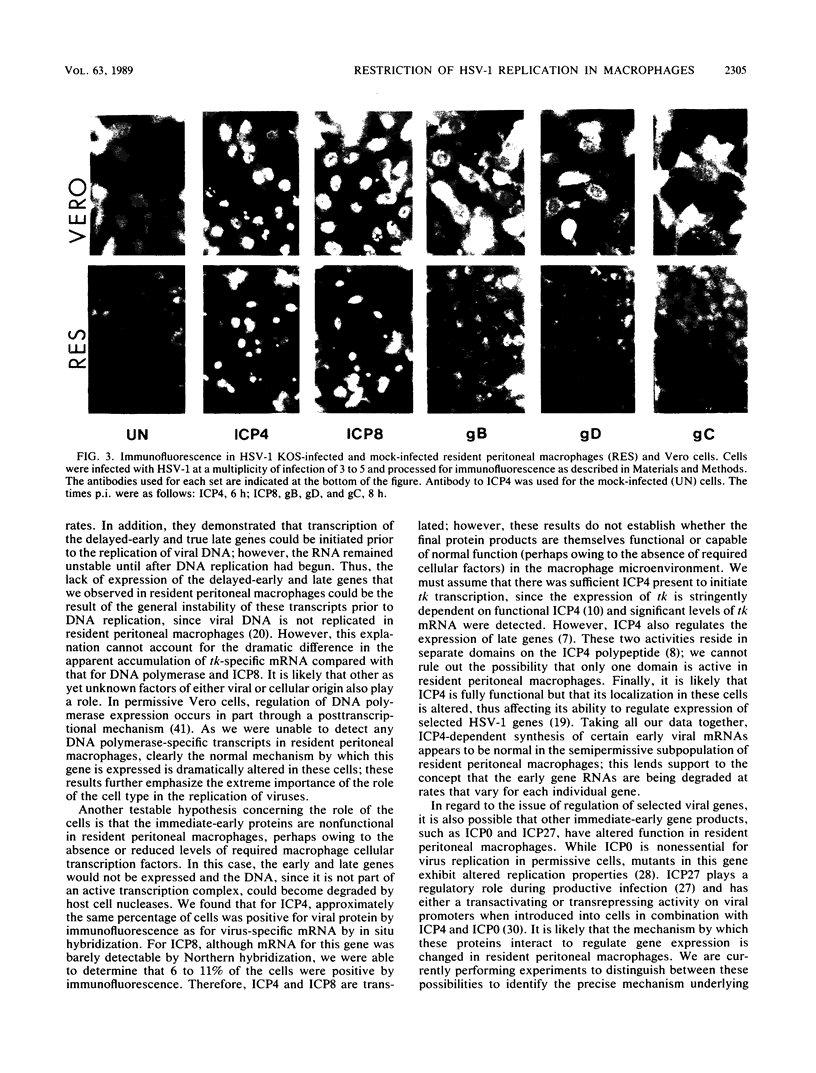
Abstract
Mononuclear phagocytes exhibit different patterns of intrinsic resistance to herpes simplex virus type 1 (HSV-1) that are related to the heterogeneity of macrophage populations and may reflect the particular differentiation or maturation state of the macrophages. In this study, we characterized the molecular basis for the block in HSV-1 replication in resident peritoneal macrophages from B6C3F1 mice. Infected resident peritoneal macrophages were analyzed for the presence of virus-specific mRNA by Northern (RNA) blotting and in situ hybridization and for proteins by immunofluorescence. The data were compared with those obtained in HSV-1-infected permissive Vero cells. The immediate-early genes ICP4, ICP0, ICP22, and ICP27 were transcribed in resident peritoneal macrophages, as was the early gene tk. Virus-specific mRNA for the major DNA-binding protein ICP8 was barely detectable, and that for another early gene, the viral DNA polymerase, was not detected. In addition, transcripts for the delayed-early gene glycoprotein D and the true late gene glycoprotein C (gC) were not detectable in resident peritoneal macrophages. In situ hybridization and immunofluorescence studies confirmed that transcripts and proteins for the immediate-early and some early HSV-1 genes were present. These data also established that 14% of the resident peritoneal macrophages were positive for RNA and polypeptide specific for the immediate-early gene ICP4 and that 7 to 11% were positive for RNA or polypeptides specific for the early genes tk and ICP8. The fact that only a few cells expressed viral products emphasizes the heterogeneity that exists even in this relatively homogeneous resident peritoneal macrophage population. Consistent with the Northern blot analysis, no RNA specific for the early DNA polymerase gene or the late gC gene was detected by in situ hybridization nor could the polypeptide for the gC gene be seen by immunofluorescence. Thus, while early transcriptional events were initiated in some resident peritoneal macrophages, there was a block in replication localized at the level of expression of the early to delayed-early viral genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Costa R. H., Holland L. E., Wagner E. K. Characterization of herpes simplex virus type 1 RNA present in the absence of de novo protein synthesis. J Virol. 1980 Apr;34(1):9–27. doi: 10.1128/jvi.34.1.9-27.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. L., Smith A. L., Morahan P. S. Effect of inapparent murine hepatitis virus infections on macrophages and host resistance. J Leukoc Biol. 1986 May;39(5):559–565. doi: 10.1002/jlb.39.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domke-Opitz I., Poberschin P., Mittnacht S., Kirchner H. Role of interferon in persistent infection of macrophages with herpes simplex virus. Virology. 1987 Aug;159(2):306–311. doi: 10.1016/0042-6822(87)90468-5. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Everett R. D. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 1987 Jul;6(7):2069–2076. doi: 10.1002/j.1460-2075.1987.tb02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J Immunol Methods. 1983 Dec 16;65(1-2):137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Senechek D., Rice S. A., Smith J. L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987 Feb;61(2):276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary K., Connor J. R., Morahan P. S. Comparison of herpes simplex virus type 1 DNA replication and virus production in murine bone marrow-derived and resident peritoneal macrophages. J Gen Virol. 1985 May;66(Pt 5):1123–1129. doi: 10.1099/0022-1317-66-5-1123. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Connor J. R., Leary K. R. Viruses and the versatile macrophage. Br Med Bull. 1985 Jan;41(1):15–21. doi: 10.1093/oxfordjournals.bmb.a072017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Schaffer P. A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987 Mar;61(3):829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Sekulovich R. E., Leary K. The alpha protein ICP0 does not appear to play a major role in the regulation of herpes simplex virus gene expression during infection in tissue culture. Nucleic Acids Res. 1987 Feb 11;15(3):905–919. doi: 10.1093/nar/15.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M. Intrinsic resistance to viral infection. Mouse macrophage restriction of herpes simplex virus replication. J Immunol. 1988 Oct 15;141(8):2740–2748. [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit M. F., Tenney D. J., Rothstein J. L., Morahan P. S. Effect of macrophage activation on resistance of mouse peritoneal macrophages to infection with herpes simplex virus types 1 and 2. J Gen Virol. 1988 Aug;69(Pt 8):1999–2010. doi: 10.1099/0022-1317-69-8-1999. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Sandri-Goldin R. M. Evidence that transcriptional control is the major mechanism of regulation for the glycoprotein D gene in herpes simplex virus type 1-infected cells. J Virol. 1988 Apr;62(4):1474–1477. doi: 10.1128/jvi.62.4.1474-1477.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub P., Domke I., Kirchner H., Jacobsen H., Panet A. Synthesis of herpes simplex virus proteins and nucleic acids in interferon-treated macrophages. Virology. 1986 Apr 30;150(2):411–418. doi: 10.1016/0042-6822(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Streicher H. Z., Joynt R. J. HTLV-III/LAV and the monocyte/macrophage. JAMA. 1986 Nov 7;256(17):2390–2391. [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Tenney D. J., Morahan P. S. Effects of differentiation of human macrophage-like U937 cells on intrinsic resistance to herpes simplex virus type 1. J Immunol. 1987 Nov 1;139(9):3076–3083. [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager D. R., Coen D. M. Analysis of the transcript of the herpes simplex virus DNA polymerase gene provides evidence that polymerase expression is inefficient at the level of translation. J Virol. 1988 Jun;62(6):2007–2015. doi: 10.1128/jvi.62.6.2007-2015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]






