Abstract
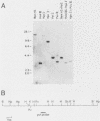
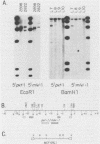
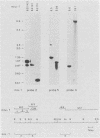
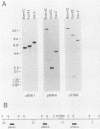
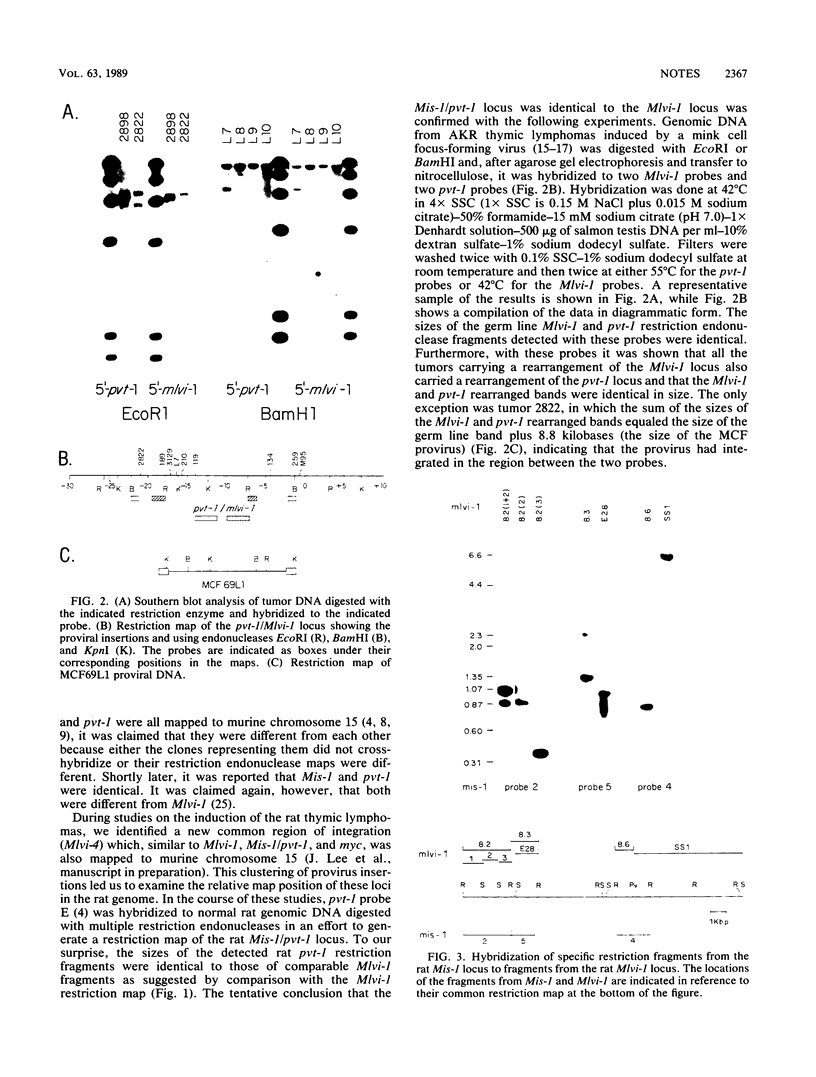
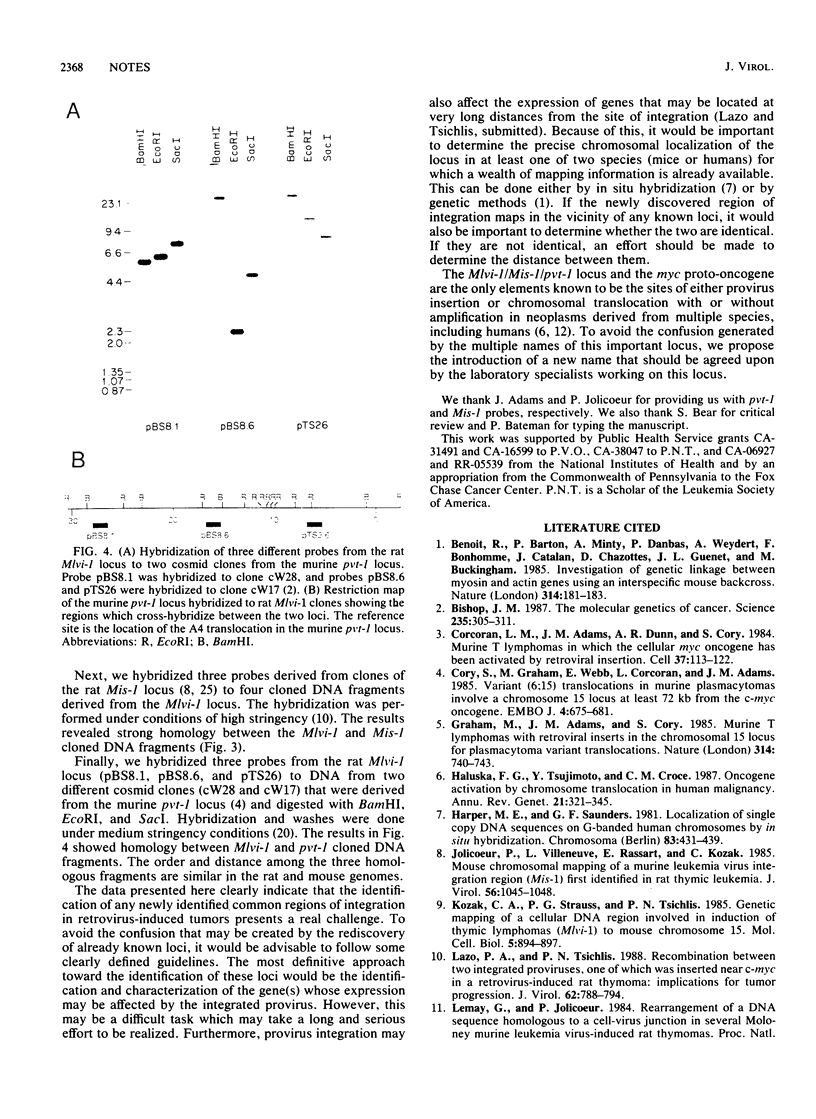
Mlvi-1 defines a locus of proviral integration in rat thymomas induced by Moloney murine leukemia virus. pvt-1/Mis-1 represents an independently identified locus which becomes rearranged either by chromosomal translocation in murine plasmacytomas or by provirus insertion in retrovirus-induced murine and rat thymic lymphomas. Although it had been claimed that pvt-1/Mis-1 and Mlvi-1 represent two different loci, we present here evidence showing that they are identical. This finding demonstrates the need for rigorous characterization of any newly identified common regions of integration in retrovirus-induced neoplasms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Graham M., Webb E., Corcoran L., Adams J. M. Variant (6;15) translocations in murine plasmacytomas involve a chromosome 15 locus at least 72 kb from the c-myc oncogene. EMBO J. 1985 Mar;4(3):675–681. doi: 10.1002/j.1460-2075.1985.tb03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M., Adams J. M., Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. 1985 Apr 25-May 1Nature. 314(6013):740–743. doi: 10.1038/314740a0. [DOI] [PubMed] [Google Scholar]
- Haluska F. G., Tsujimoto Y., Croce C. M. Oncogene activation by chromosome translocation in human malignancy. Annu Rev Genet. 1987;21:321–345. doi: 10.1146/annurev.ge.21.120187.001541. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Villeneuve L., Rassart E., Kozak C. Mouse chromosomal mapping of a murine leukemia virus integration region (Mis-1) first identified in rat thymic leukemia. J Virol. 1985 Dec;56(3):1045–1048. doi: 10.1128/jvi.56.3.1045-1048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Strauss P. G., Tsichlis P. N. Genetic mapping of a cellular DNA region involved in induction of thymic lymphomas (Mlvi-1) to mouse chromosome 15. Mol Cell Biol. 1985 Apr;5(4):894–897. doi: 10.1128/mcb.5.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A., Tsichlis P. N. Recombination between two integrated proviruses, one of which was inserted near c-myc in a retrovirus-induced rat thymoma: implications for tumor progression. J Virol. 1988 Mar;62(3):788–794. doi: 10.1128/jvi.62.3.788-794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay G., Jolicoeur P. Rearrangement of a DNA sequence homologous to a cell-virus junction fragment in several Moloney murine leukemia virus-induced rat thymomas. Proc Natl Acad Sci U S A. 1984 Jan;81(1):38–42. doi: 10.1073/pnas.81.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., Rabbitts T. H. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987 Jul;6(7):1959–1965. doi: 10.1002/j.1460-2075.1987.tb02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Gilbert D. J., Taylor B. A., Jenkins N. A., Copeland N. G. Common sites of viral integration in lymphomas arising in AKXD recombinant inbred mouse strains. Oncogene Res. 1987;2(1):33–48. [PubMed] [Google Scholar]
- Neil J. C., Forrest D. Mechanisms of retrovirus-induced leukaemia: selected aspects. Biochim Biophys Acta. 1987 Apr 20;907(1):71–91. doi: 10.1016/0304-419x(87)90019-9. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Fleissner E., Lonial H., Koehne C. F., Reicin A. Early clonality and high-frequency proviral integration into the c-myc locus in AKR leukemias. J Virol. 1985 Aug;55(2):500–503. doi: 10.1128/jvi.55.2.500-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Traganos F. Changes in thymocyte proliferation at different stages of viral leukemogenesis in AKR mice. J Immunol. 1986 Jan;136(2):720–727. [PubMed] [Google Scholar]
- O'Donnell P. V., Woller R., Chu A. Stages in development of mink cell focus-inducing (MCF) virus-accelerated leukemia in AKR mice. J Exp Med. 1984 Sep 1;160(3):914–934. doi: 10.1084/jem.160.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Buckler C. E. A preferred region for integration of Friend murine leukemia virus in hematopoietic neoplasms is closely linked to the Int-2 oncogene. J Virol. 1986 Dec;60(3):1156–1158. doi: 10.1128/jvi.60.3.1156-1158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Kozak C. A. Cellular DNA region involved in induction of thymic lymphomas (Mlvi-2) maps to mouse chromosome 15. Mol Cell Biol. 1984 May;4(5):997–1000. doi: 10.1128/mcb.4.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Kozak C., Robinson H. L. Dsi-1, a region with frequent proviral insertions in Moloney murine leukemia virus-induced rat thymomas. J Virol. 1987 Apr;61(4):1164–1170. doi: 10.1128/jvi.61.4.1164-1170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemur R., Monczak Y., Rassart E., Kozak C., Jolicoeur P. Identification of a new common provirus integration site in gross passage A murine leukemia virus-induced mouse thymoma DNA. Mol Cell Biol. 1987 Jan;7(1):512–522. doi: 10.1128/mcb.7.1.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve L., Rassart E., Jolicoeur P., Graham M., Adams J. M. Proviral integration site Mis-1 in rat thymomas corresponds to the pvt-1 translocation breakpoint in murine plasmacytomas. Mol Cell Biol. 1986 May;6(5):1834–1837. doi: 10.1128/mcb.6.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E., Adams J. M., Cory S. Variant (6 ; 15) translocation in a murine plasmacytoma occurs near an immunoglobulin kappa gene but far from the myc oncogene. Nature. 1984 Dec 20;312(5996):777–779. doi: 10.1038/312777a0. [DOI] [PubMed] [Google Scholar]
- Wirschubsky Z., Tsichlis P., Klein G., Sumegi J. Rearrangement of c-myc, pim-1 and Mlvi-1 and trisomy of chromosome 15 in MCF- and Moloney-MuLV-induced murine T-cell leukemias. Int J Cancer. 1986 Nov 15;38(5):739–745. doi: 10.1002/ijc.2910380518. [DOI] [PubMed] [Google Scholar]