Abstract
Type II restriction and modification (R-M) genes have been described as selfish because they have been shown to impose selection for the maintenance of the plasmid that encodes them. In our experiments, the type I R-M system EcoKI does not behave in the same way. The genes specifying EcoKI are, however, normally residents of the chromosome and therefore our analyses were extended to monitor the deletion of chromosomal genes rather than loss of plasmid vector. If EcoKI were to behave in the same way as the plasmid-encoded type II R-M systems, the loss of the relevant chromosomal genes by mutation or recombination should lead to cell death because the cell would become deficient in modification enzyme and the bacterial chromosome would be vulnerable to the restriction endonuclease. Our data contradict this prediction; they reveal that functional type I R-M genes in the chromosome are readily replaced by mutant alleles and by alleles encoding a type I R-M system of different specificity. The acquisition of allelic genes conferring a new sequence specificity, but not the loss of the resident genes, is dependent on the product of an unlinked gene, one predicted [Prakash-Cheng, A., Chung, S. S. & Ryu, J. (1993) Mol. Gen. Genet. 241, 491–496] to be relevant to control of expression of the genes that encode EcoKI. Our evidence suggests that not all R-M systems are evolving as “selfish” units; rather, the diversity and distribution of the family of type I enzymes we have investigated require an alternative selective pressure.
Restriction systems are widespread in bacteria. A classical restriction system comprises a restriction endonuclease and a modification enzyme (R-M system); the modification enzyme recognizes the same nucleotide sequence as the endonuclease and, by methylating a particular base, or bases, within each target sequence, renders the DNA refractory to endonucleolytic attack by the cognate restriction enzyme. Restriction systems are so varied in their organization and behavior that they have been subdivided into at least three types (for reviews see refs. 1 and 2). A common explanation for the observed diversity of sequence specificity is that R-M systems have evolved under selective pressure to protect bacteria from infection by foreign DNA. This hypothesis has been challenged by Naito et al. (3) who question “whether such a cellular defense hypothesis explains the extreme diversity and specificity of sequence recognition.”
Two groups have provided convincing support for the idea that type II R-M systems can serve to maintain the presence of the plasmid that encodes them (3, 4). Their data are consistent with the concept of a restriction enzyme as a toxin that is normally neutralized by an antidote, the modification enzyme. When a cell continues to divide following the loss of the plasmid, the level of antidote may become insufficient to protect all the restriction targets from the endonuclease, and consequently, cells lacking the plasmid will die (see ref. 5). Type II R-M genes that behave in this way may be regarded as “selfish” because there would be direct selection for their retention rather than their presence being maintained as the consequence of the advantage they confer on the host bacterium (3).
Type II R-M systems are the simplest, and each system consists of two separate enzymes, an endonuclease and a methyltransferase which show no notable similarity in amino acid sequence despite the fact that they recognize the same target sequence.
In contrast, a purified type I R-M system comprises one oligomeric complex that is both a restriction endonuclease and a modification methyltransferase, but in vivo the large complex coexists with a smaller one endowed with only modification activity. A single subunit confers sequence specificity to both complexes and, because any change in specificity affects both restriction and modification, type I systems are more suited to the evolution of new specificities than type II. Consistent with this, type I R-M systems are readily grouped into families within which members such as EcoKI and EcoBI are distinguished by their specificity subunits, which endow each system with specificity for a different target sequence.
Very early studies, in which the genes encoding EcoKI and EcoBI were identified by mutation, showed that mutants defective in both restriction and modification were more common than those defective only in restriction (6). These results already cast doubt on the general applicability of the toxin–antidote hypothesis; nevertheless, this hypothesis is of sufficient interest to warrant testing.
In this paper, we question whether the restriction genes encoding a type I R-M system (EcoKI) can serve to maintain the presence of a plasmid that encodes them. Our experiments demonstrate that a plasmid encoding EcoKI is not maintained any better than one encoding only the modification enzyme. The genes specifying EcoKI are normally resident on the bacterial chromosome rather than within a plasmid; therefore, we extended our analyses to the genes in their usual chromosomal location. If EcoKI were to behave in the same way as the plasmid-encoded type II R-M systems, the loss of the relevant chromosomal genes by mutation or recombination should lead to cell death because the cell would become deficient in modification enzyme and the bacterial chromosome would be vulnerable to the restriction endonuclease. Our experiments contradict this prediction; they reveal that functional type I R-M genes in the chromosome are readily replaced by mutant alleles and by alleles encoding a type I R-M system of different specificity.
It has been shown that at least one gene in Escherichia coli strain C is essential for the acquisition of an F′ encoding the type I R-M system of E. coli K-12 (7). We investigate the effect of the hsdC mutation on the acquisition and loss of hsd genes by P1 transduction.
MATERIALS AND METHODS
Bacterial Strains, Phages, and Plasmids.
C600 (8) was the standard rK+mK+ E. coli K-12 strain; NM854 and NM840 are rK−mK− derivatives of C600 and C600 gyrA, respectively. In NM840, a deletion extends from the BamHI site in hsdM to the adjacent BamHI site in hsdR (9), and in NM854 the DNA between the BamHI site in hsdM and a SalI site in hsdS has been replaced by a DNA fragment including the gene from Tn903 that confers resistance to kanamycin (M.O., unpublished data). NM679 is a derivative of W3110 deleted for a segment of DNA that includes the entire hsd coding sequence and the flanking mcr and mrr genes (10). Mutations in hsd genes, and the alternative hsdS gene of the EcoDI system, were introduced into the bacterial chromosome via λhsd phages to make strains NM840, -854, -515, and -807 (see Table 1). λNM1048, an att– int– cI857 phage including hsdM and S and most of hsdR, was used to mediate the transfer of hsd markers from one bacterial strain to another (11). The E. coli C strains JR300 and JR302 have been described by Prakash-Cheng et al. (7). JR300 is wild type, and JR302 is an hsdC recA KanR derivative in which recA was introduced by conjugation from an E. coli K-12 donor. The E. coli C strains (Table 1) were descendants of either JR300 or NM820, a derivative of JR302 in which the recA allele has been replaced by the recA+ gene from a λrecA phage.
Table 1.
Bacterial strains used in transduction experiments
| NM+ number | Background* | Relevant genotype | Use |
|---|---|---|---|
| 679 | K-12 | Δ(mcr hsd mrr) | Donor |
| 840 | K-12 | Δ(hsdM-R) | Donor |
| 854 | K-12 | Δ(hsdSM)hsd∷kan* | Donor |
| 515 | K-12 | hsdSD | Donor |
| 807 | K-12 | hsdSDhsdR | Donor |
| 815 | K-12 | dnaC325 zjj∷Tn10 | Recipient |
| 814 | K-12 | hsdR dnaC325 zjj∷Tn10 | Recipient |
| 824 | C | (hsdS+M+R−)†dnaC325 zjj∷Tn10 | Recipient |
| 827, 865 | C | (hsd+)†dnaC325 zjj∷Tn10 | Recipient |
| 822, 866 | C | (hsdS+M+R−)†dnaC325 zjj∷Tn10 hsdC | Recipient |
| 839, 846 | C | (hsd+)†dnaC325 zjj∷Tn10 hsdC | Recipient |
See text for further details.
hsd region from E. coli K-12.
The temperature-sensitive dnaC strains used as recipients in P1 transduction experiments (see Table 1) were made by P1-mediated transfer of dnaC325 zjj::Tn10 from either TCP48, an hsd+ donor (12), or NM814, an hsdR− donor. The latter permitted the transfer of hsd genes to an hsdC derivative of E. coli C. The defective hsdR gene was then replaced by P1 transduction using dnaC+ hsd+ donors. dnaC+ hsd+ derivatives (NM831 and NM845) from different transductions were used as recipients to make NM839 and NM846 by the reintroduction of dnaC325 zjj::Tn10 from the hsd+ donor TCP48.
An att+ imm21 prophage including hsdM and S (λNM1324) was used to provide EcoKI-specific modification in the donor strains used for determining cotransduction frequencies.
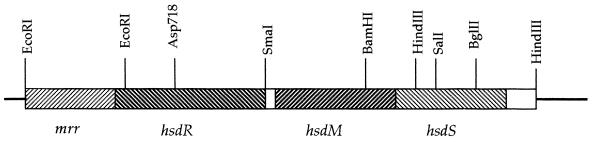
Plasmid phsd+ has the entire hsdRMS region of E. coli K-12 in pBR322 (Fig. 1) and was made in two steps from plasmids pBg3 and pRH3 (11). Plasmid pRH1 includes hsdM, most of hsdR, and part of hsdS within a 5.7-kb EcoRI–HindIII fragment. This plasmid was cut with HindIII, and a 1.9-kb HindIII fragment from pRH3, which includes the 3′ part of hsdS, was inserted to complete the coding sequence for the modification enzyme. The resulting hsdM+S+ plasmid was cut at the EcoRI site, and the 2-kb EcoRI fragment from pBg3, which encodes the 5′ region of hsdR, was inserted to complete the coding sequence for EcoKI. phsd+ confers a restriction and modification-proficient (rK+mK+) phenotype to an hsd-deletion host. The phsdR− plasmid differs from phsd+ only by a missense mutation (A619V) in hsdR (13). The 1.2-kb Asp718–SmaI fragment containing this mutation was used to replace the wild-type sequence in phsd+. phsdR− confers a modification-proficient phenotype (rK−mK+) to an hsd-deletion host.
Figure 1.
The plasmid phsd+ includes hsdRMS in pBR322. The relevant restriction sites are indicated. Plasmid phsdR− is identical except for a missense mutation in hsdR (see Materials and Methods).
Media and Microbial Techniques.
Media and general methods (14) and tests for estimating restriction and modification (15) have been described. P1kc was used for all transductions (16) as detailed by Miller (17).
The quantification of plasmid maintenance in the absence of selection was done by a repeated batch culture method in which the cells were serially diluted 106-fold and incubated for 12 h in L broth at 37°C with aeration (18). The generation time under these conditions was 35 min.
Analysis and Ligation of DNA.
Restriction enzymes and T4 DNA ligase were purchased from Boehringer Mannheim or New England Biolabs and used as recommended by the suppliers. Ligations were done by standard methods (19).
RESULTS
Plasmid Maintenance.
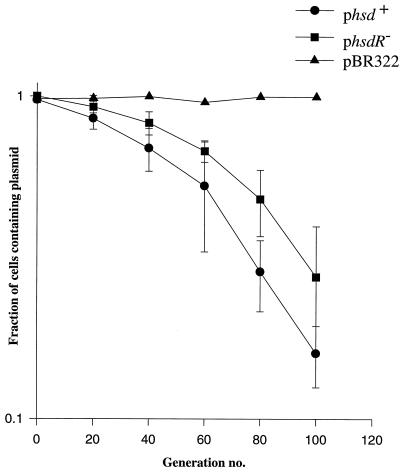
The effect of a functional EcoKI complex on plasmid maintenance was determined using two derivatives of pBR322 of similar size and genetic content. Both included mrr and the three hsd genes required to encode EcoKI, but in one plasmid (phsd+) all three hsd genes were of wild-type sequence, and in the other (phsdR−) a missense mutation inactivated the hsdR gene (Fig. 1). The two plasmids were used to transform a strain (NM679) deficient in both restriction and modification (rK−mK−), and the transformed bacteria acquired the expected phenotypes; those transformed with phsd+ were restriction and modification proficient (rK+mK+), and those transformed with phsdR− were only modification proficient (rK−mK+). Transformed cells were serially subcultured in the absence of ampicillin, and samples were plated on L agar, then replica plated to L agar and L agar supplemented with ampicillin (100 μg/ml) and methicillin (100 μg/ml), to assess what fraction of the cells maintained the resident plasmid. The experiments compared the hsd-deletion strain (NM679) transformed with phsd+ or phsdR− with the same strain transformed with the vector pBR322. Although pBR322 is very well maintained in the absence of ampicillin, plasmids including hsd genes were not (Fig. 2). These data provide no evidence to support the idea that a plasmid encoding EcoKI is maintained better than an hsdR− derivative conferring an rK−mK+ phenotype.
Figure 2.
The maintenance of plasmids phsd+ and phsdR− in NM679 compared with pBR322 (see Materials and Methods). The fractions of cells retaining plasmids are plotted on a log scale. Those for phsd+ and phsdR− were based on the data of three experiments and those for pBR322 on two. No standard deviations are shown for the latter because there was negligible deviation.
An alternative way of monitoring the presence of phsd+ is from the efficiency of plating (e.o.p.) of unmodified phage λ. Restriction is a particularly sensitive test because the e.o.p. of unmodified λ will rise from less than 10−3 to 10−2 when only 1% of the cells have lost the plasmid encoding the R-M system. The e.o.p. of λ on a fresh overnight culture of NM679 transformed with phsd+, and grown in the presence of ampicillin, failed to restrict λ as effectively as an E. coli strain with a single copy of chromosomal hsd genes. The e.o.p. of unmodified phage rose quickly when the culture was grown in the absence of ampicillin. This increased e.o.p. was shown to result from plasmid loss rather than plasmid change.
The plasmid encoding EcoKI was not maintained any better than its hsdR− derivative; therefore, no evidence was obtained to support the idea that EcoKI can serve to maintain the presence of the plasmid that encodes it.
The Loss of Chromosomal hsd Genes by Transduction.
Contiguous, chromosomally located genes encode the polypeptides (HsdR, M, and S) of EcoKI, an oligomeric complex that is both a restriction endonuclease and a modification methyltransferase. The HsdM and HsdS polypeptides also associate to form a second complex that has only the modification activity. HsdS confers both complexes with the same sequence specificity. In a wild-type cell, the level of the modification enzyme may be much higher than that of the multifunctional complex (13, 20), and both the concentrations and ratios of the two complexes may be affected when the hsd genes are on a multicopy plasmid rather than the chromosome.
The efficiency with which chromosomal hsd genes are deleted by P1-mediated transduction was monitored, therefore, for hsdR+ (rK+mK+) and hsdR− (rK−mK+) strains. It should be noted that the Dar protein of phage P1 protects bacterial DNA transferred within phage capsids from restriction by the EcoKI system of the recipient strain (21). Nevertheless, unless stated otherwise, in case this protection is incomplete the donor strains in all of the P1 transduction experiments reported in this paper were lysogenic for a λhsdMS phage to provide K-specific modification of the donor DNA. Since the modification genes within the prophage are located at the attachment site of phage λ, they cannot be cotransduced with dnaC.
P1 lysates were made on hsd-deletion mutants and used to transduce dnaC− recipient strains; the mutation in dnaC results in a temperature-sensitive lethal phenotype. DnaC+ transductants were selected at 42°C and scored for cotransduction of the hsd deletion and Tn10, a marker on the opposite side of dnaC from hsd. (Table 2).
Table 2.
Transduction by rK−mK− donors
| Donor* | Recipient | Percent of transductants with donor phenotypes
|
|
|---|---|---|---|
| DnaC+ rK−mK− | DnaC+ TetS | ||
| NM854 | NM815 (rK+mK+) | 52 | 96 |
| NM854 | NM814 (rK−mK+) | 50 | 96 |
| NM840 | NM815 (rK+mK+) | 43 | 96 |
| NM840 | NM814 (rK−mK+) | 56 | 94 |
| NM679 | NM815 (rK+mK+) | 98 (94)† | 96 (90)† |
| NM679 | NM814 (rK−mK+) | 100 (95)† | 97 (92)† |
The order of genes on the chromosome is hsdS hsdM hsdR dnaC Tn10. Cotransduction frequencies are based on a minimum of 50 colonies.
See Table 1 for identification of hsd deletion. The hsdSM deletion in NM854 is tagged by a gene conferring resistance to kanamycin. The other deletions were recognized by their restriction and modification phenotype.
Donor strain for data within brackets was without λhsdM+S+ prophage and therefore mK−.
The first two mutants used have deletions confined to the hsd genes. Approximately 50% of the DnaC+ transductants acquired the Hsd− phenotype of the donor, but rather than assess whether the minor difference (56 vs. 43%) observed for the deletion in NM840 might be significant, a third and more extensive deletion was used to increase the cotransduction frequencies, making minor discrepancies easier to detect. Two P1 lysates were used, one of which was made on the deletion mutant in the absence of the λhsd prophage; the unmodified P1 lysate was restricted around 10-fold by the rK+ recipient. Cotransduction frequencies using the third deletion were in excess of 90% irrespective of the hsdR genotype of the recipient or the modification phenotype of the donor strain.
The results of the present experiments, which assess the loss of R-M genes by genetic recombination, contrast with those obtained for certain type II R-M systems based on the loss of plasmids encoding R-M systems. (3, 4). The presence of functional type I restriction genes in the recipient had little, if any, effect on the probability with which a DnaC+ transductant acquired the defect in the modification genes.
Concomitant Loss and Gain of hsd Genes.
The diversity of type I R-M systems encoded by alleles at the hsd locus is consistent with the ready acquisition of new specificities by either gene replacement or rearrangement (see refs. 1 and 2). In the following experiments, P1 transduction was used to quantify the efficiency with which the hsd genes encoding EcoKI were replaced by those conferring a different specificity. As in the previous experiments, selection was made for DnaC+ transductants. Substitution of the hsd genes specifying the EcoDI system for the resident ones encoding EcoKI occurred in 40/70 (57%) of the DnaC+ transductants when both the donor and recipient were hsdR+ (NM515 and NM815 in Table 1) and in 35/70 (50%) of the DnaC+ transductants when both strains were hsdR− (NM807 and NM814).
This result is consistent not only with the easy loss of functional hsd genes but with the efficient acquisition of hsd genes encoding a restriction system with a different specificity. The data provide no evidence to support the idea that it is more difficult to acquire a new specificity when the R-M systems are proficient in restriction than it is when they are defective in restriction; this is so despite the fact that the acquisition of the genes encoding a new system requires that the recipient DNA becomes modified before any restriction activity has the opportunity to cut the host DNA and that the HsdR polypeptides of the two systems are functionally indistinguishable.
A Functional hsdC Gene Is Required for the Efficient Transduction of hsd Genes Conferring a New Specificity.
E. coli C has no known R-M system and lacks approximately 18 kb of DNA in the region of the hsd locus of E. coli K-12 (22). A derivative of E. coli C has been isolated that has lost the ability to serve as a recipient for an F′ encoding a functional EcoKI enzyme (7). This mutant strain is postulated to be defective in a function essential for the control of the restriction phenotype, and the relevant gene was designated hsdC (C for control). How HsdC functions is unknown, but it is presumed that it postpones the production of the restriction enzyme until the modification enzyme has protected the DNA.
The effect of hsdC on the transfer of hsd genes by P1 transduction was determined. These experiments can only be done in the E. coli C background in which the hsdC mutation was isolated. The nature and precise location of hsdC have not been reported. To provide homology for recombination, E. coli C recipients were used that included the hsd region of E. coli K-12, but with a mutation in hsdR to confer a rK−mK+ phenotype.
Transduction of the new specificity (hsdSD) was monitored in an hsdC+ E. coli C strain (NM824). The hsdS gene of the EcoDI system was acquired irrespective of whether the donor was hsdR+ or hsdR− (Table 3). In both cases, the frequency of transductants with the donor phenotype (12–16%) was less than that (50–57%) for an E. coli K-12 recipient, but low frequencies of cotransfer are characteristic of transduction experiments between E. coli and Salmonella (23) and might be anticipated if the DNA sequences of two strains of E. coli are sufficiently dissimilar.
Table 3.
The effect of hsdC on the acquisition of new R-M systems
| Donor | Recipient | Percent of transductants with donor phenotypes
|
|
|---|---|---|---|
| DnaC+ HsdSD | DnaC+ TetS | ||
| NM515 (rD+mD+) | NM824 (rK−mK+) | 16 | 80 |
| NM807 (rD−mD+) | NM824 (rK−mK+) | 12 | 71 |
| NM515 (rD+mD+) | NM822 (rK−mK+, HsdC−) | 0* | 71 |
| NM807 (rD−mD+) | NM822 (rK−mK+, HsdC−) | 12 | 72 |
| NM807 (rD−mD+) | NM846 (rK+mK+, HsdC−) | 1† | 89 |
The order of the genes on the bacterial chromosome is hsdS hsdM hsdR dnaC Tn10. Cotransduction frequencies are based on at least 50 colonies.
0 in 100 transductants.
One rD−mD+ and two rK−mK+ transductants; the former requires entry of the hsdSD gene and the potential for the production of EcoDI before recombination occurs.
The experiment was extended to an hsdC rK−mK+ derivative of E. coli C (NM822). The hsdC mutation reduced the efficiency with which a new specificity was acquired in the presence, but not in the absence, of a functional hsdR gene (Table 3). No hsdC recipient among 100 DnaC+ transductants acquired a functional R-M system conferring the new specificity if the donor encoded a functional R-M system, although transductants were isolated that had an hsdR+ gene and hence became proficient in restriction for the resident modification system of E. coli K-12. Even the acquisition of functional modification genes with the specificity of EcoDI from the rD− donor NM807 was impeded if the recipient was rK+mK+. The subunits of EcoKI and EcoDI can be exchanged and, presumably, when an hsdC− cell acquired a donor fragment including hsdSD and hsdM, complementation resulted in an active EcoDI restriction enzyme before the host DNA could be modified. Restriction of the recipient DNA would prevent the recovery of rD−mD+ recombinants.
Our data show that the function identified by Prakash-Cheng et al (7) is required for the efficient incorporation of chromosomal genes encoding EcoDI, a closely related type I restriction system conferring a different specificity from EcoKI.
Does hsdC Affect the Loss of hsd Genes As Well As the Acquisition?
If HsdC affects the establishment of the restriction-proficient phenotype, it could do so by antagonizing the endonucleolytic activity of EcoKI or destabilizing the EcoKI complex.
It is possible that it is the HsdC function that makes the chromosomally encoded type I R-M system behave differently from the type II systems. For this reason, the effect of the hsdC genotype on the loss of functional hsd genes was determined in an hsdR+ and hsdR− background. The hsdC mutation did not impose a barrier to the loss of functional hsd genes (Table 4, compare NM846 and NM839 with NM827 and NM865 and with NM822 and NM866). The cotransduction frequencies varied from 23 to 50%, but this variation was seen even when the recipients were identical in terms of their hsd genotypes, e.g., NM846 and NM839 or NM827 and NM865. An obvious explanation of this variability is that the extent of the homology in the hsd dnaC Tn10 region is not the same in each hybrid recipient. The upper figure (50%) is the same as that obtained in experiments where the recipient was a K-12 strain (see Table 2). The minor variations do not obscure the conclusion that the hsdC mutation had little or no effect on the ease with which the hsd+ genes were deleted. The major difference in the behavior between EcoKI and some type II R-M systems cannot be attributed to the protective role of the product of hsdC.
Table 4.
The effect of hsdC on the loss of hsd genes
| Donor
|
Recipient
|
Percent of transductants with donor phenotype* DnaC+ Hsd− | ||
|---|---|---|---|---|
| Strain | Phenotype | Strain | Phenotype | |
| NM840 | rK−mK− | NM827 | rK+mK+ | 23 (14/61) |
| NM865 | rK+mK+ | 50 (15/30) | ||
| NM840 | rK−mK− | NM822 | rK−mK+, HsdC− | 36 (20/55) |
| NM866 | rK−mK+, HsdC− | 50 (12/24) | ||
| NM840 | rK−mK− | NM846 | rK+mK+, HsdC− | 25 (21/80) |
| NM840 | rK−mK− | NM839 | rK+mK+, HsdC− | 50 (27/54) |
| NM515 | rD+mD+ | NM846 | rK+mK+, HsdC− | — (0/85)† |
| NM515 | rD+mD+ | NM839 | rK+mK+, HsdC− | — (0/26)† |
Percent with DnaC+ TetS phenotype varied from 66% to 84%.
Absence of rD+mD+ transductants confirms HsdC− phenotype of recipient.
DISCUSSION
Early experiments demonstrating the chromosomal location of hsd genes in E. coli strains other than K-12, and in Salmonella serotypes, relied on the P1-mediated transfer of hsd genes from one strain to another including the substitution of the resident genes by alleles conferring new specificities (24, 25). The success of these experiments is consistent with the efficient transfer of hsd genes, but the consequences of functional restriction genes in either the donor or the recipient were not investigated; rather, these experiments laid the initial evidence for a diversity of sequence specificities conferred by allelic hsd genes.
Allelic variability is one of the most striking features of type I R-M systems, and even within the laboratory, type I R-M systems with new specificities have been found by chance as well as by experimental design. The specificity subunit of a type I R-M enzyme includes two target recognition domains (TRDs) each specifying one component of the target sequence. This organization of domains makes the specificity subunits well suited to the generation of new specificities either as the consequence of new combinations of TRDs or of minor changes in the spacing between the TRDs (26, 27). Both the bipartite and asymmetrical nature of the target sequence of type I systems offer more scope for diversity of sequence specificity than the symmetrical recognition sequences of type II R-M systems.
Efficient changes of specificity in vivo require that the parental genes are replaced; a new restriction system is acquired at the expense of the old ones. Our experiments do not detect any barrier to the acquisition of new specificities; rather, for type IA systems there must be an effective means of preventing a bacterium from becoming restriction proficient until the newly formed modification enzyme has protected unmethylated target sequences within the chromosomal DNA. This is also true for EcoRI24I, a plasmid-encoded type IC R-M system, although in this case the mechanism of control is not dependent on HsdC (28). The EcoKI and EcoRI24I systems must modify unmethylated target sequences despite the fact that only hemimethylated DNA is a good substrate for the cognate methyltransferase, but for EcoKI there is evidence that recipient bacteria do not become restriction proficient until many generations after the acquisition of the hsd genes (29).
Previous experiments have failed to find evidence for transcriptional control of expression of hsd genes (7, 9). Posttranslational control at the level of subunit assembly is likely to be a critical factor (30). The production of EcoKI by the addition of two HsdR subunits to the modification enzyme is an obvious step that could be susceptible to influence by proteases and chaperone-like activities. Although the means of delaying the production of an active restriction endonuclease remains to be shown, it is already clear that a very effective mechanism exists which enables bacteria to generate and recruit genes specifying alternative restriction systems. The type I R-M systems, therefore, are especially suited to the evolution of new specificities not simply because restriction and modification specificities change concomitantly but because these complex systems have coevolved with their host in such a way that cell death is prevented when a new specificity arises.
Naito et al. (3) have argued that the “cellular defense” hypothesis cannot readily explain the evolution of “rare cutter” enzymes because long recognition sequences are unlikely to be present in many bacterial viruses. The target sequences of type I R-M systems are all long, usually 7 bp but sometimes 6 and occasionally 8 bp, whereas those of type II systems are commonly 4–6 bp and only occasionally 8 bp. Nevertheless, most of the known type I systems have been detected by their restriction of phages.
Currently, there is no evidence to support the idea that type I R-M genes are selfish; rather, the extremely high, intraspecific, allelic variability is consistent with frequency-dependent selection for diversity of specificity (see refs. 31–33). The common explanation for the diversity has been one in which bacteriophages have imposed selection for bacteria with rare specificities; bacteria colonizing a new habitat are anticipated to have an advantage if they possess an R-M system with a rare specificity (31). This advantage would be short-lived (34) but could act on different strains of the same species. Cellular defense remains a possible current role for some R-M systems. An alternative, nonselfish role has been suggested in which restriction stimulates, or in some way modulates, recombination (see refs. 33 and 35 and references therein). The effect of type I R-M systems on recombination remains to be evaluated, although some evidence implicates restriction in the incorporation of small DNA segments into a recipient genome following P1-mediated transduction (35, 36).
Analysis of the hsd genes found in natural isolates of E. coli indicates unusual allelic diversity (33). Furthermore, as the nucleotide sequences for additional bacterial genomes are determined, a striking feature is the number of putative R-M systems of all types (see, for example, ref. 37). No single model may explain the maintenance and evolution of the present diversity of types and sequence specificities of R-M systems. The full roles that restriction plays in the lives of prokaryotes remain to be understood.
Acknowledgments
We are grateful to our colleagues for helpful suggestions and support, most particularly David Leach, Svetlana Makovets, Lynn Powell, and Annette Titheradge. We thank Karen Witherspoon for her patient help in preparing the manuscript and the Medical Research Council for a research grant.
ABBREVIATIONS
- TRD
target recognition domain
- R-M
restriction–modification
Note Added in Proof
The hsd plasmids used to test plasmid maintenance (Fig. 2) include mrr in addition to the hsd genes. The deletion of mrr from phsd+ had little or no effect on the maintenance of this plasmid and the Mrr protein is not, therefore, a major case of the poor maintenance of phsd+ (unpublished data).
References
- 1.Barcus V A, Murray N E. In: Population Genetics of Bacteria. Baumberg S, Young J P W, Saunders S R, Wellington E M H, editors. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 31–58. [Google Scholar]
- 2.Redaschi N, Bickle T A. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, Curtiss R, Ingrahm J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, D.C.: Am. Soc. Microbiol.; 1996. pp. 773–781. [Google Scholar]
- 3.Naito T, Kusano K, Kobayashi I. Science. 1995;267:897–898. doi: 10.1126/science.7846533. [DOI] [PubMed] [Google Scholar]
- 4.Kulakauskas S, Lubys A, Ehrlich S D. J Bacteriol. 1995;177:3451–3454. doi: 10.1128/jb.177.12.3451-3454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarmolinsky M B. Science. 1995;267:836–837. doi: 10.1126/science.7846528. [DOI] [PubMed] [Google Scholar]
- 6.Wood W B. J Mol Biol. 1966;16:118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- 7.Prakash-Cheng A, Chung S S, Ryu J. Mol Gen Genet. 1993;241:491–496. doi: 10.1007/BF00279890. [DOI] [PubMed] [Google Scholar]
- 8.Appleyard R K. Genetics. 1954;39:440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loenen W A M, Daniel A S, Braymer H D, Murray N E. J Mol Biol. 1987;198:159–170. doi: 10.1016/0022-2836(87)90303-2. [DOI] [PubMed] [Google Scholar]
- 10.King G, Murray N E. Mol Microbiol. 1995;16:769–777. doi: 10.1111/j.1365-2958.1995.tb02438.x. [DOI] [PubMed] [Google Scholar]
- 11.Sain B, Murray N E. Mol Gen Genet. 1980;180:35–46. doi: 10.1007/BF00267350. [DOI] [PubMed] [Google Scholar]
- 12.Masters M, Paterson T, Popplewell A G, Owen-Hughes T, Pringle J H, Begg K J. Mol Gen Genet. 1989;216:475–483. doi: 10.1007/BF00334393. [DOI] [PubMed] [Google Scholar]
- 13.Webb J L, King G, Ternent D, Titheradge A J B, Murray N E. EMBO J. 1996;15:2003–2009. [PMC free article] [PubMed] [Google Scholar]
- 14.Murray N E, Brammar W J, Murray K. Mol Gen Genet. 1977;150:53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- 15.Fuller-Pace F V, Cowan G M, Murray N E. J Mol Biol. 1985;186:65–75. doi: 10.1016/0022-2836(85)90257-8. [DOI] [PubMed] [Google Scholar]
- 16.Lennox E S. Virology. 1995;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller J M. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. pp. 203–205. [Google Scholar]
- 18.Nordstrom K. In: Plasmids: A Practical Approach. Hardy K G, editor. Oxford: IRL Press; 1993. pp. 1–38. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Weiserova M, Janscak P, Benada O, Zinkevich V E, Glover S W, Firman K. Nucleic Acids Res. 1993;21:373–370. doi: 10.1093/nar/21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iida S, Streigg M B, Bickle T A, Arber W. Virology. 1987;157:156–166. doi: 10.1016/0042-6822(87)90324-2. [DOI] [PubMed] [Google Scholar]
- 22.Daniel A S, Fuller-Pace F V, Legge D M, Murray N E. J Bacteriol. 1988;170:1775–1782. doi: 10.1128/jb.170.4.1775-1782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayssiguier C, Thaler D S, Radman M. Nature (London) 1992;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 24.Arber W, Wauters-Willems D. Mol Gen Genet. 1970;180:203–207. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- 25.Bullas L R, Colson R C, Neufeld B. J Bacteriol. 1980;141:275–292. doi: 10.1128/jb.141.1.275-292.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller-Pace F V, Bullas L R, Delius H, Murray N E. Proc Natl Acad Sci USA. 1985;81:6095–6099. doi: 10.1073/pnas.81.19.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price C J, Shepherd C W, Bickle T A. EMBO J. 1987;6:1493–1497. doi: 10.1002/j.1460-2075.1987.tb02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulik E M, Bickle T A. J Mol Biol. 1996;264:891–906. doi: 10.1006/jmbi.1996.0685. [DOI] [PubMed] [Google Scholar]
- 29.Prakash-Cheng A, Ryu R. J Bacteriol. 1993;175:4905–4906. doi: 10.1128/jb.175.15.4905-4906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dryden D T F, Cooper L P, Thorpe P H, Byron O. Biochemistry. 1997;36:1065–1076. doi: 10.1021/bi9619435. [DOI] [PubMed] [Google Scholar]
- 31.Levin B R. Philos Trans R Soc London B. 1988;319:459–472. doi: 10.1098/rstb.1988.0059. [DOI] [PubMed] [Google Scholar]
- 32.Sharp P M, Kelleher J E, Daniel A S, Cowan G M, Murray N E. Proc Natl Acad Sci USA. 1992;89:9836–9840. doi: 10.1073/pnas.89.20.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barcus V A, Titheradge A J B, Murray N E. Genetics. 1995;140:1187–1197. doi: 10.1093/genetics/140.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korona R, Levin B R. Evolution. 1993;47:556–575. doi: 10.1111/j.1558-5646.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 35.Milkman R. Genetics. 1997;146:745–750. doi: 10.1093/genetics/146.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKane M, Milkman R. Genetics. 1995;139:35–43. doi: 10.1093/genetics/139.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, et al. Nature (London) 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]