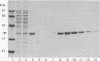
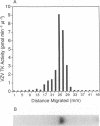
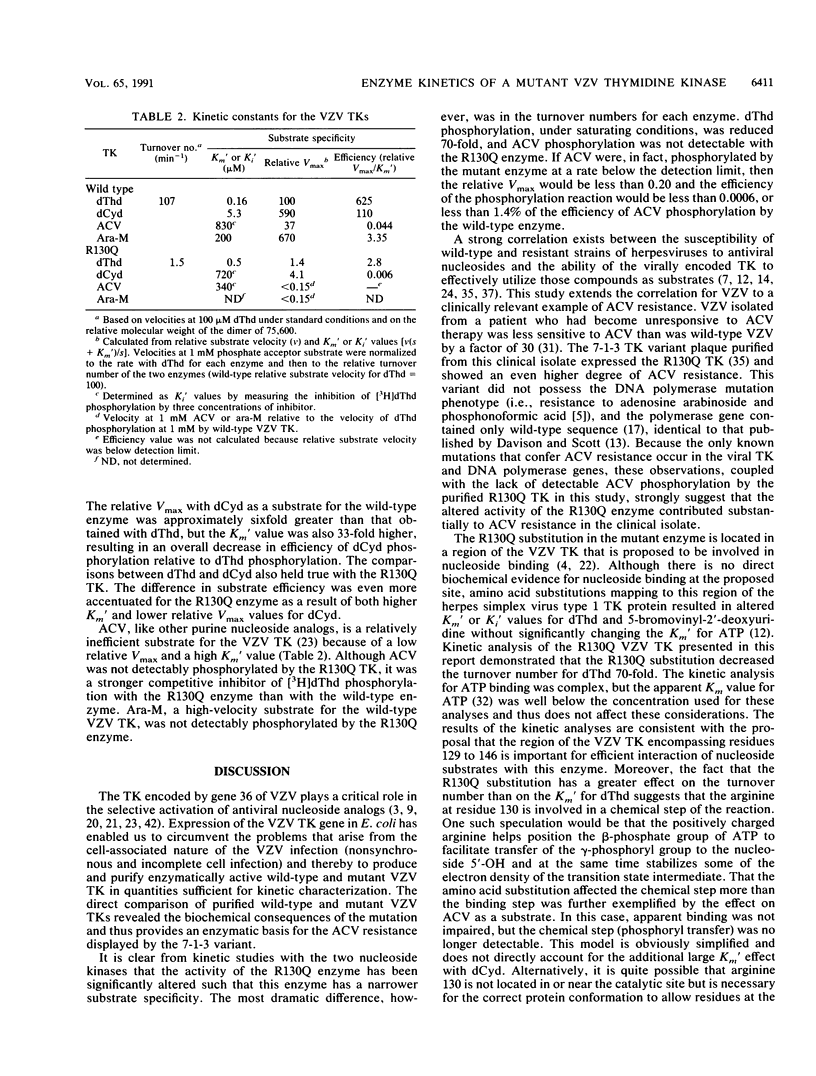
Abstract
Varicella-zoster virus (VZV) encodes a thymidine kinase (EC 2.7.2.21) which phosphorylates several antiviral nucleoside analogs, including acyclovir (ACV). A mutation in the VZV thymidine kinase coding sequence, resulting in an arginine-to-glutamine substitution at amino acid residue 130 (R130Q), is associated with clinical resistance to ACV. We have expressed the wild-type and the mutant enzymes in bacteria and have studied the kinetic characteristics of the purified enzymes. The arginine-to-glutamine substitution resulted in decreased catalytic activity and altered substrate specificity. The most striking effect was a decrease in the rates of nucleoside phosphorylation to less than 2% of the rates with the wild-type enzyme. This was accompanied by increased apparent Km values for thymidine and deoxycytidine. ACV was not detectably phosphorylated by the R130Q enzyme but still competed with thymidine for the enzyme. The inability of the R130Q enzyme to catalyze the phosphorylation of ACV correlates with resistance to ACV noted with a clinical isolate of VZV.
Full text
PDF

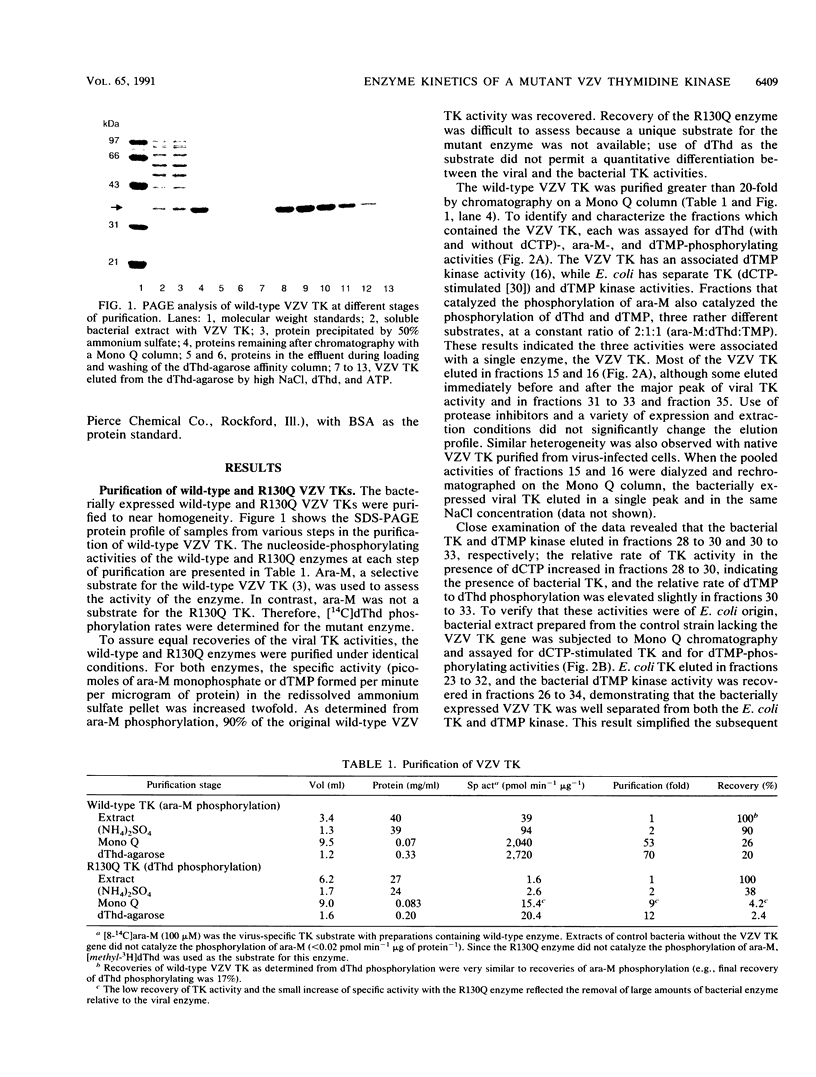
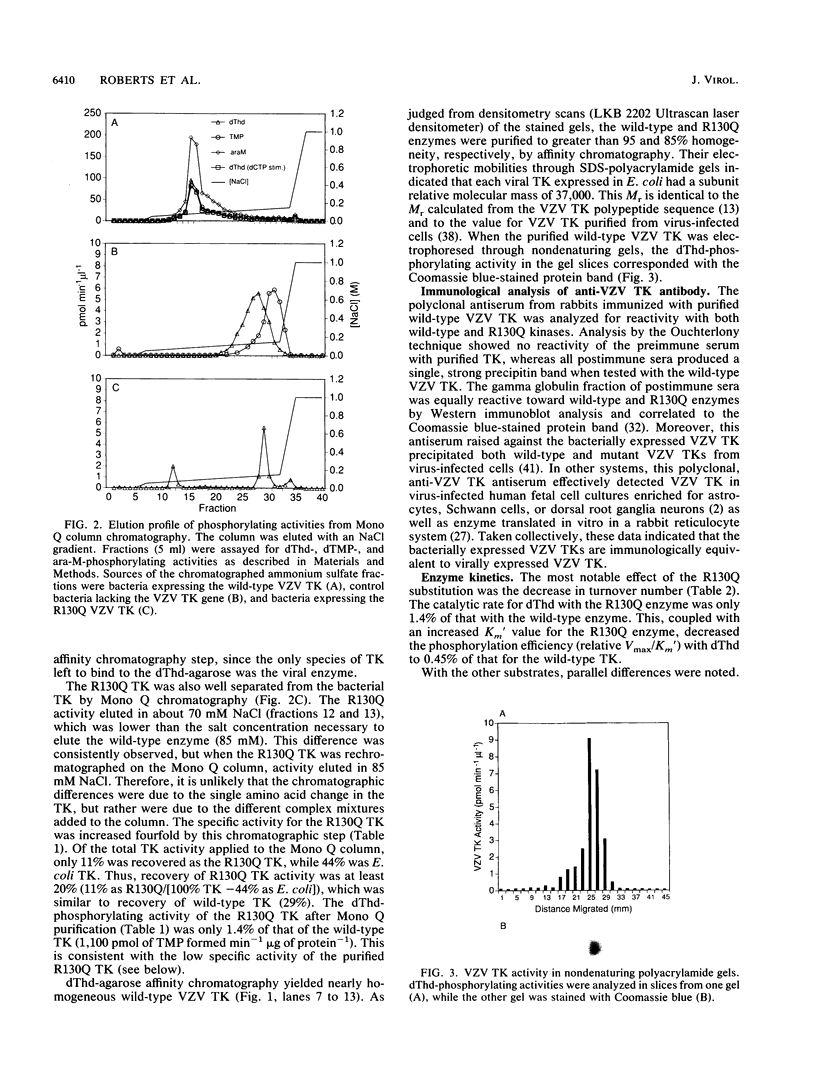


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assouline J. G., Levin M. J., Major E. O., Forghani B., Straus S. E., Ostrove J. M. Varicella-zoster virus infection of human astrocytes, Schwann cells, and neurons. Virology. 1990 Dec;179(2):834–844. doi: 10.1016/0042-6822(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Averett D. R., Koszalka G. W., Fyfe J. A., Roberts G. B., Purifoy D. J., Krenitsky T. A. 6-Methoxypurine arabinoside as a selective and potent inhibitor of varicella-zoster virus. Antimicrob Agents Chemother. 1991 May;35(5):851–857. doi: 10.1128/aac.35.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam N. K., Veerisetty V., Gentry G. A. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J Gen Virol. 1990 Dec;71(Pt 12):2979–2987. doi: 10.1099/0022-1317-71-12-2979. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Fyfe J. A., Noblin J. E., Elion G. B. Selection and preliminary characterization of acyclovir-resistant mutants of varicella zoster virus. Am J Med. 1982 Jul 20;73(1A):383–386. doi: 10.1016/0002-9343(82)90128-0. [DOI] [PubMed] [Google Scholar]
- Burnette T. C., Koszalka G. W., Krenitsky T. A., De Miranda P. Metabolic disposition and pharmacokinetics of the antiviral agent 6-methoxypurine arabinoside in rats and monkeys. Antimicrob Agents Chemother. 1991 Jun;35(6):1165–1173. doi: 10.1128/aac.35.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Dutschman G., De Clercq E., Jones A. S., Rahim S. G., Verhelst G., Walker R. T. Differential affinities of 5-(2-halogenovinyl)-2'-deoxyuridines for deoxythymidine kinases of various origins. Mol Pharmacol. 1981 Jul;20(1):230–233. [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Ellis M. N., Keller P. M., Fyfe J. A., Martin J. L., Rooney J. F., Straus S. E., Lehrman S. N., Barry D. W. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother. 1987 Jul;31(7):1117–1125. doi: 10.1128/aac.31.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Mallavia L. P. Deoxypyrimidine nucleoside metabolism in varicella-zoster virus-infected cells. J Virol. 1978 Feb;25(2):510–517. doi: 10.1128/jvi.25.2.510-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. A., Berger T. G., Fikrig S., Becherer P., Moohr J. W., Stanat S. C., Biron K. K. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990 Feb 1;112(3):187–191. doi: 10.7326/0003-4819-112-3-187. [DOI] [PubMed] [Google Scholar]
- Karlström A. R., Källander C. F., Abele G., Larsson A. Acyclic guanosine analogs as substrates for varicella-zoster virus thymidine kinase. Antimicrob Agents Chemother. 1986 Jan;29(1):171–174. doi: 10.1128/aac.29.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Fyfe J. A., Beauchamp L., Lubbers C. M., Furman P. A., Schaeffer H. J., Elion G. B. Enzymatic phosphorylation of acyclic nucleoside analogs and correlations with antiherpetic activities. Biochem Pharmacol. 1981 Nov 15;30(22):3071–3077. doi: 10.1016/0006-2952(81)90495-0. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase. Microbiol Sci. 1985 Dec;2(12):369–375. [PubMed] [Google Scholar]
- Koszalka G. W., Averett D. R., Fyfe J. A., Roberts G. B., Spector T., Biron K., Krenitsky T. A. 6-N-substituted derivatives of adenine arabinoside as selective inhibitors of varicella-zoster virus. Antimicrob Agents Chemother. 1991 Jul;35(7):1437–1443. doi: 10.1128/aac.35.7.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S. F., Suzutani T., Powell K. L., Purifoy D. J., Honess R. W. Analysis of mutations in the thymidine kinase genes of drug-resistant varicella-zoster virus populations using the polymerase chain reaction. J Gen Virol. 1991 Mar;72(Pt 3):623–630. doi: 10.1099/0022-1317-72-3-623. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Mahalingam R., Cabirac G., Wellish M., Gilden D., Vafai A. In-vitro synthesis of functional varicella zoster and herpes simplex viral thymidine kinase. Virus Genes. 1990 Jul;4(2):105–120. doi: 10.1007/BF00678403. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- Pahwa S., Biron K., Lim W., Swenson P., Kaplan M. H., Sadick N., Pahwa R. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA. 1988 Nov 18;260(19):2879–2882. [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer M. H., Inchauspe G., Biron K. K., Waters D. J., Straus S. E., Ostrove J. M. Molecular analysis of the pyrimidine deoxyribonucleoside kinase gene of wild-type and acyclovir-resistant strains of varicella-zoster virus. J Gen Virol. 1988 Oct;69(Pt 10):2585–2593. doi: 10.1099/0022-1317-69-10-2585. [DOI] [PubMed] [Google Scholar]
- Shigeta S., Mori S., Yokota T., Konno K., De Clercq E. Characterization of a varicella-zoster virus variant with altered thymidine kinase activity. Antimicrob Agents Chemother. 1986 Jun;29(6):1053–1058. doi: 10.1128/aac.29.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Ogino T., Yamanishi K., Takahashi M. Immunochemical characterization of pyrimidine kinase induced by varicella-zoster virus. J Gen Virol. 1985 Feb;66(Pt 2):221–229. doi: 10.1099/0022-1317-66-2-221. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Ogino T., Yamanishi K., Takahashi M. Thymidine kinase with altered substrate specificity of acyclovir resistant varicella-zoster virus. Biken J. 1986 Mar;29(1):7–10. [PubMed] [Google Scholar]
- Spector T., Cleland W. W. Meanings of Ki for conventional and alternate-substrate inhibitors. Biochem Pharmacol. 1981 Jan 1;30(1):1–7. doi: 10.1016/0006-2952(81)90277-x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Konno K., Mori S., Shigeta S., Kumagai M., Watanabe Y., Machida H. Mechanism of selective inhibition of varicella zoster virus replication by 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Mol Pharmacol. 1989 Aug;36(2):312–316. [PubMed] [Google Scholar]