Abstract
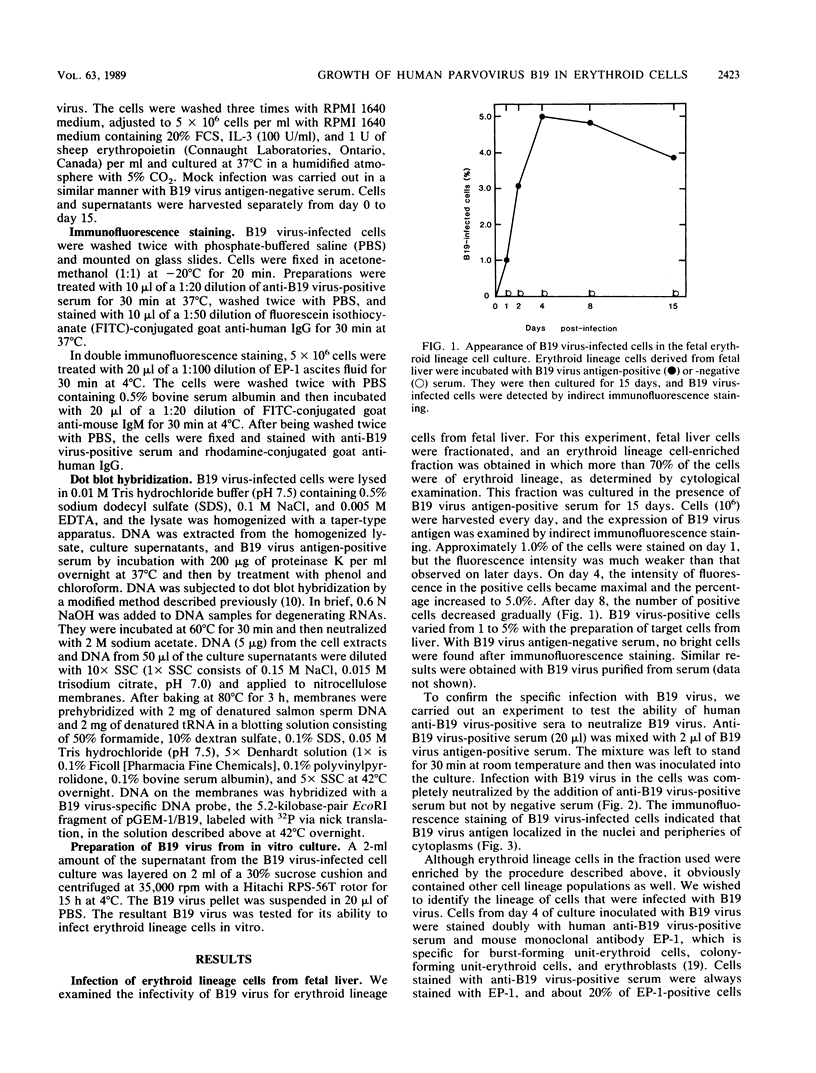
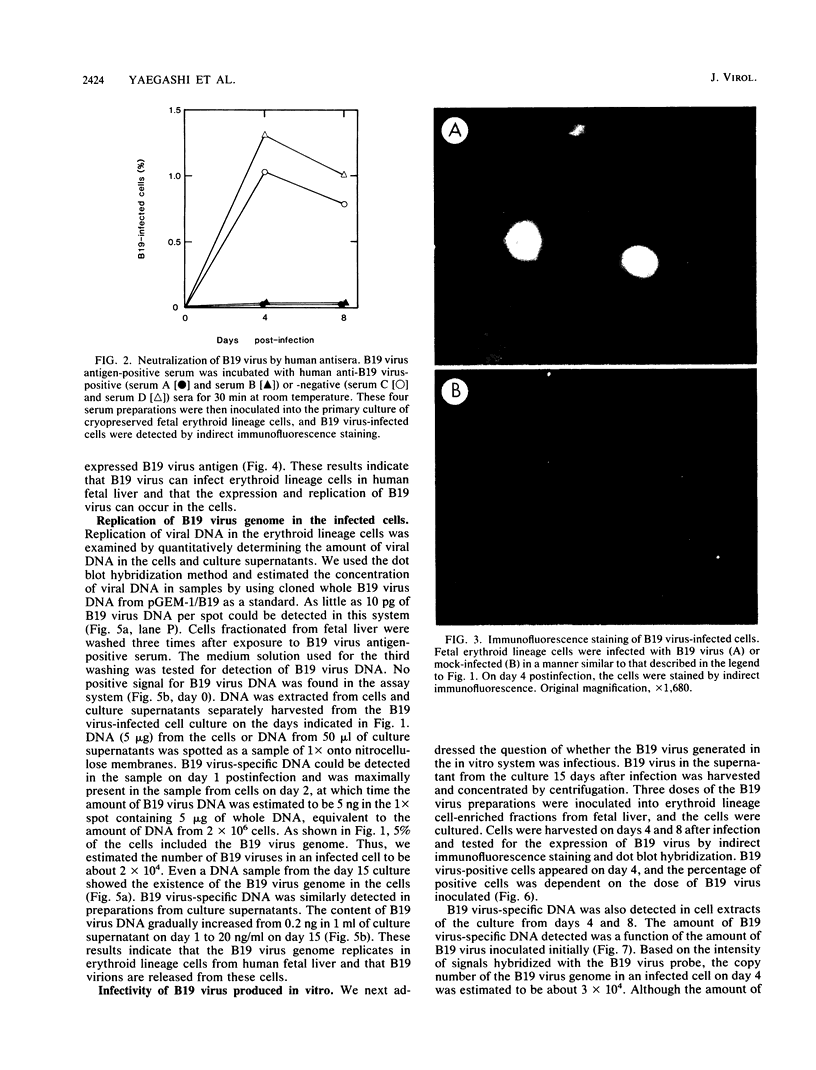
Erythroid lineage cells derived from fetal liver were demonstrated to be target cells for human parvovirus B19 infection. B19 virus antigen-positive serum was inoculated into primary cultures containing erythroid lineage cells enriched from fetal liver. The B19 virus antigen was detected on about 5% of cells in the culture by immunofluorescence staining, and the stained cells were identified as erythroid lineage cells by double staining with anti-B19 virus-positive serum and anti-erythroid lineage monoclonal antibody. The immunofluorescence staining study also revealed that the B19 virus antigen localized in the nucleus and the periphery of cytoplasm. We also detected B19 virus DNA, which was generated by replication in the infected cells, not only in the cells but also in the culture supernatants, in which the amount of B19 DNA increased depending on the period of culture, indicating that the cells infected with B19 virus produced B19 virus and released it into the medium. The ability of B19 virus released into the medium to infect fetal erythroid lineage cells was demonstrated quantitatively. Because of the absence of any cytopathic effect of B19 virus during culture periods of at least 15 days, this culture system should be useful in the study of B19 virus replication and in vitro generation of B19 virus. In addition, the present study may contribute to a better understanding of the pathogenesis of hydrops fetalis, which is probably associated with B19 virus infection during pregnancy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand A., Gray E. S., Brown T., Clewley J. P., Cohen B. J. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med. 1987 Jan 22;316(4):183–186. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Lewis E., Kidd I. M., Hall S. M., Cohen B. J. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984 Aug;93(1):85–93. doi: 10.1017/s0022172400060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Buckley M. M., Clewley J. P., Jones V. E., Puttick A. H., Jacoby R. K. Human parvovirus infection in early rheumatoid and inflammatory arthritis. Ann Rheum Dis. 1986 Oct;45(10):832–838. doi: 10.1136/ard.45.10.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- McPherson R. A., Ginsberg H. S., Rose J. A. Adeno-associated virus helper activity of adenovirus DNA binding protein. J Virol. 1982 Nov;44(2):666–673. doi: 10.1128/jvi.44.2.666-673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori J., Beattie P., Melton D. W., Cohen B. J., Clewley J. P. Structure and mapping of the DNA of human parvovirus B19. J Gen Virol. 1987 Nov;68(Pt 11):2797–2806. doi: 10.1099/0022-1317-68-11-2797. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet. 1985 Feb 23;1(8426):422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- Saarinen U. M., Chorba T. L., Tattersall P., Young N. S., Anderson L. J., Palmer E., Coccia P. F. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986 May;67(5):1411–1417. [PubMed] [Google Scholar]
- Shiraishi H., Wong D., Purcell R. H., Shirachi R., Kumasaka E., Numazaki Y. Antibody to human parvovirus in outbreak of erythema infectiosum in Japan. Lancet. 1985 Apr 27;1(8435):982–983. doi: 10.1016/s0140-6736(85)91752-0. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol. 1988 Aug;62(8):3059–3063. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- Woernle C. H., Anderson L. J., Tattersall P., Davison J. M. Human parvovirus B19 infection during pregnancy. J Infect Dis. 1987 Jul;156(1):17–20. doi: 10.1093/infdis/156.1.17. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Brice M., Rabinovitch P. S., Papayannopoulou T., Stamatoyannopoulos G. Monoclonal antibodies detecting antigenic determinants with restricted expression on erythroid cells: from the erythroid committed progenitor level to the mature erythroblast. Blood. 1984 Jun;63(6):1376–1384. [PubMed] [Google Scholar]
- Young N., Harrison M., Moore J., Mortimer P., Humphries R. K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest. 1984 Dec;74(6):2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]







