Abstract
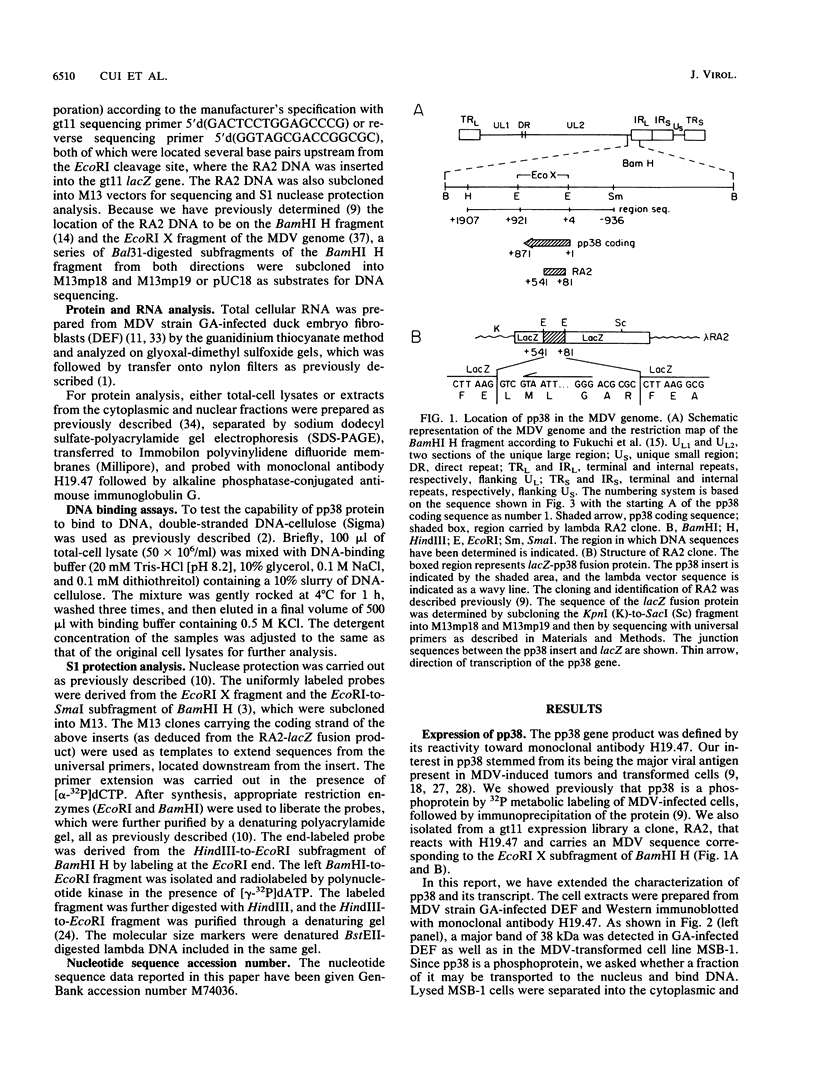
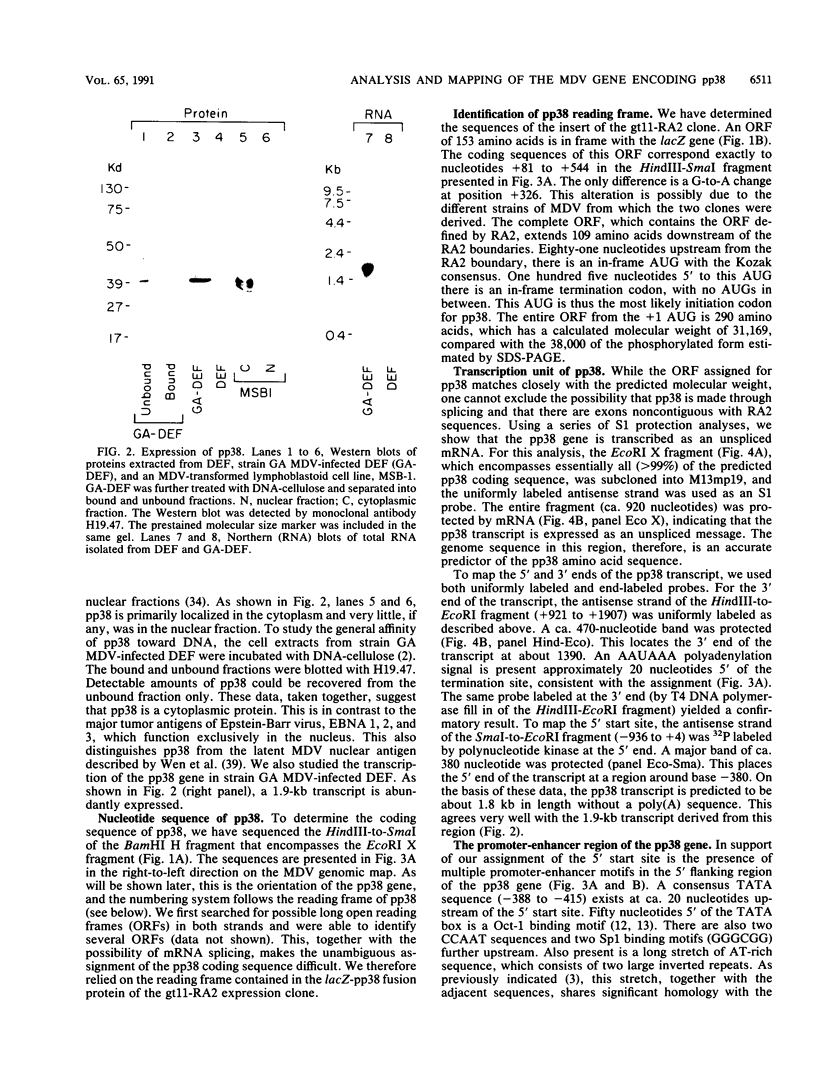
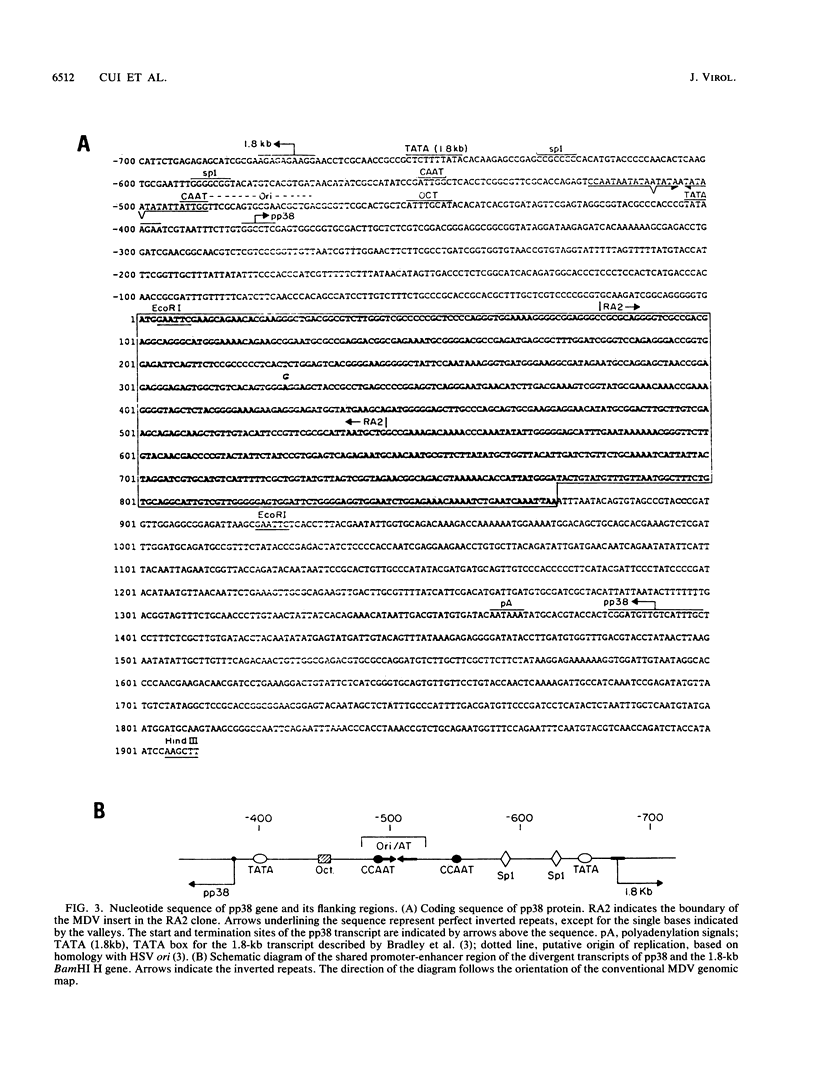
The gene encoding a Marek's disease virus (MDV) pp38 phosphoprotein has been identified, sequenced, and localized to the BamHI H fragment to the left of the putative MDV origin of replication. The open reading frame was defined by sequencing of a lacZ-pp38 fusion protein gene from a lambda gt11 expression library. The entire open reading frame is 290 amino acids long and codes for a protein with a calculated molecular weight of 31,169, compared with the size of 38 kDa of the phosphorylated form estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. S1 nuclease protection analysis showed that the pp38 gene is transcribed leftward as an unspliced mRNA. On the basis of transcriptional mapping studies, the pp38 transcript is predicted to be about 1.8 kb in length without a poly(A) sequence. Its promoter-enhancer region overlaps that of the major rightward BamHI H 1.8-kb transcript implicated in tumor induction. This region contains Oct-1, Sp1, and CCAAT motifs as well as the putative origin of replication. The pp38 protein is the only presently known antigen that is consistently associated with the transformation state. It may play a significant role in MDV transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucher P., Koning A., Privalsky M. L. The avian erythroblastosis virus erbA oncogene encodes a DNA-binding protein exhibiting distinct nuclear and cytoplasmic subcellular localizations. J Virol. 1988 Feb;62(2):534–544. doi: 10.1128/jvi.62.2.534-544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Lancz G., Tanaka A., Nonoyama M. Loss of Marek's disease virus tumorigenicity is associated with truncation of RNAs transcribed within BamHI-H. J Virol. 1989 Oct;63(10):4129–4135. doi: 10.1128/jvi.63.10.4129-4135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster A. E., Scott S. D., Sanderson M. J., Boursnell M. E., Ross N. L., Binns M. M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988 Aug;69(Pt 8):2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- Chen X. B., Velicer L. F. Multiple bidirectional initiations and terminations of transcription in the Marek's disease virus long repeat regions. J Virol. 1991 May;65(5):2445–2451. doi: 10.1128/jvi.65.5.2445-2451.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Velicer L. F. Structure and complete nucleotide sequence of the Marek's disease herpesvirus gp57-65 gene. J Virol. 1988 Jul;62(7):2373–2379. doi: 10.1128/jvi.62.7.2373-2379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Cui Z. Z., Yan D., Lee L. F. Marek's disease virus gene clones encoding virus-specific phosphorylated polypeptides and serological characterization of fusion proteins. Virus Genes. 1990 Apr;3(4):309–322. doi: 10.1007/BF00569038. [DOI] [PubMed] [Google Scholar]
- Eidson C. S., Schmittle S. C. Studies on acute Marek's disease. I. Characteristics of isolate GA in chickens. Avian Dis. 1968 Aug;12(3):467–476. [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Mocikat R., Zachau H. G. Sequences closely related to an immunoglobulin gene promoter/enhancer element occur also upstream of other eukaryotic and of prokaryotic genes. Nucleic Acids Res. 1986 Nov 25;14(22):8819–8827. doi: 10.1093/nar/14.22.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Wettenhall R. E., Kemp B. E. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987 Jan 15;262(2):772–777. [PubMed] [Google Scholar]
- Igarashi T., Takahashi M., Donovan J., Jessip J., Smith M., Hirai K., Tanaka A., Nonoyama M. Restriction enzyme map of herpesvirus of turkey DNA and its collinear relationship with Marek's disease virus DNA. Virology. 1987 Apr;157(2):351–358. doi: 10.1016/0042-6822(87)90277-7. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Nakajima K., Naito M., Ann S. H., Ueda S., Kato S., Hirai K. Identification of Marek's disease virus-specific antigens in Marek's disease lymphoblastoid cell lines using monoclonal antibody against virus-specific phosphorylated polypeptides. Int J Cancer. 1985 Feb 15;35(2):257–264. doi: 10.1002/ijc.2910350219. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Kung H. J., Velicer L. F. Identification of the gene encoding Marek's disease herpesvirus A antigen. J Virol. 1987 Aug;61(8):2614–2620. doi: 10.1128/jvi.61.8.2614-2620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Hayashi M., Furuichi T., Nonoyama M., Isogai E., Namioka S. The inhibitory effects of oligonucleotides, complementary to Marek's disease virus mRNA transcribed from the BamHI-H region, on the proliferation of transformed lymphoblastoid cells, MDCC-MSB1. J Gen Virol. 1991 May;72(Pt 5):1105–1111. doi: 10.1099/0022-1317-72-5-1105. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Liu X., Witter R. L. Monoclonal antibodies with specificity for three different serotypes of Marek's disease viruses in chickens. J Immunol. 1983 Feb;130(2):1003–1006. [PubMed] [Google Scholar]
- Lee L. F., Nazerian K., Leinbach S. S., Reno J. M., Boezi J. A. Effect of phosphonoacetate on Marek's disease virus replication. J Natl Cancer Inst. 1976 Apr;56(4):823–827. doi: 10.1093/jnci/56.4.823. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie M. J., McGeoch D. J. DNA sequence analysis of an immediate-early gene region of the herpes simplex virus type 1 genome (map coordinates 0.950 to 0.978). J Gen Virol. 1982 Sep;62(Pt 1):1–15. doi: 10.1099/0022-1317-62-1-1. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Ikuta K., Naito M., Ueda S., Kato S., Hirai K. Analysis of Marek's disease virus serotype 1-specific phosphorylated polypeptides in virus-infected cells and Marek's disease lymphoblastoid cells. J Gen Virol. 1987 May;68(Pt 5):1379–1389. doi: 10.1099/0022-1317-68-5-1379. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Preston C. M., Cordingley M. G., Stow N. D. Analysis of DNA sequences which regulate the transcription of a herpes simplex virus immediate early gene. J Virol. 1984 Jun;50(3):708–716. doi: 10.1128/jvi.50.3.708-716.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Purchase H. G. Immunofluorescence in the study of Marek's disease. I. Detection of antigen in cell culture and an antigenic comparison of eight isolates. J Virol. 1969 Jun;3(6):557–565. doi: 10.1128/jvi.3.6.557-565.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Sanderson M., Scott S. D., Binns M. M., Doel T., Milne B. Nucleotide sequence and characterization of the Marek's disease virus homologue of glycoprotein B of herpes simplex virus. J Gen Virol. 1989 Jul;70(Pt 7):1789–1804. doi: 10.1099/0022-1317-70-7-1789. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L. T., Tanaka A., Nonoyama M. Identification of Marek's disease virus nuclear antigen in latently infected lymphoblastoid cells. J Virol. 1988 Oct;62(10):3764–3771. doi: 10.1128/jvi.62.10.3764-3771.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Clements J. B. Replication origins and a sequence involved in coordinate induction of the immediate-early gene family are conserved in an intergenic region of herpes simplex virus. Nucleic Acids Res. 1984 Feb 24;12(4):2061–2079. doi: 10.1093/nar/12.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter R. L. Characteristics of Marek's disease viruses isolated from vaccinated commercial chicken flocks: association of viral pathotype with lymphoma frequency. Avian Dis. 1983 Jan-Mar;27(1):113–132. [PubMed] [Google Scholar]
- Witter R. L., Silva R. F., Lee L. F. New serotype 2 and attenuated serotype 1 Marek's disease vaccine viruses: selected biological and molecular characteristics. Avian Dis. 1987 Oct-Dec;31(4):829–840. [PubMed] [Google Scholar]
- Wong S. W., Schaffer P. A. Elements in the transcriptional regulatory region flanking herpes simplex virus type 1 oriS stimulate origin function. J Virol. 1991 May;65(5):2601–2611. doi: 10.1128/jvi.65.5.2601-2611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]





