Abstract
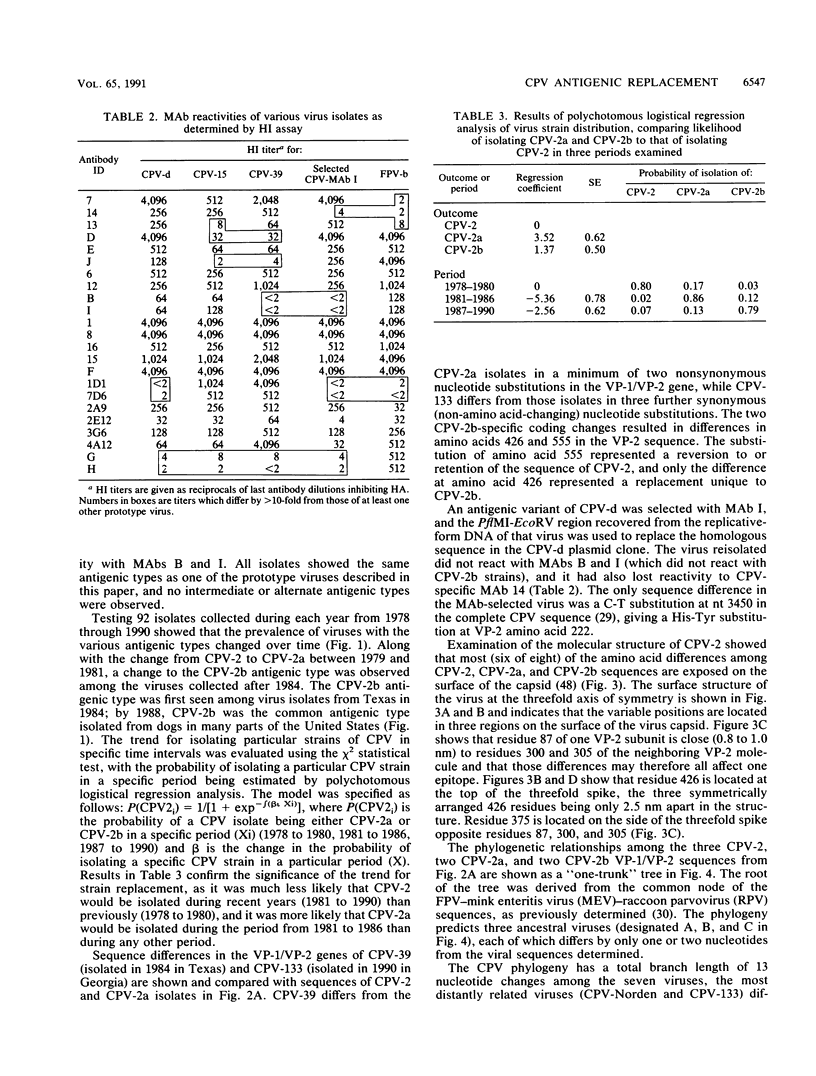
Analysis of canine parvovirus (CPV) isolates with a panel of monoclonal antibodies showed that after 1986, most viruses isolated from dogs in many parts of the United States differed antigenically from the viruses isolated prior to that date. The new antigenic type (designated CPV type 2b) has largely replaced the previous antigenic type (CPV type 2a) among virus isolates from the United States. This represents the second occurrence of a new antigenic type of this DNA virus since its emergence in 1978, as the original CPV type (CPV type 2) had previously been replaced between 1979 and 1981 by the CPV type 2a strain. DNA sequence comparisons showed that CPV types 2b and 2a differed by as few as two nonsynonymous (amino acid-changing) nucleotide substitutions in the VP-1 and VP-2 capsid protein genes. One mutation, resulting in an Asn-Asp difference at residue 426 in the VP-2 sequence, was shown by comparison with a neutralization-escape mutant selected with a non-CPV type 2b-reactive monoclonal antibody to determine the antigenic change. The mutation selected by that monoclonal antibody, a His-Tyr difference in VP-2 amino acid 222, was immediately adjacent to residue 426 in the three-dimensional structure of the CPV capsid. The CPV type 2b isolates are phylogenetically closely related to the CPV type 2a isolates and are probably derived from a common ancestor. Phylogenetic analysis showed a progressive evolution away from the original CPV type. This pattern of viral evolution appears most similar to that seen in some influenza A viruses.
Full text
PDF
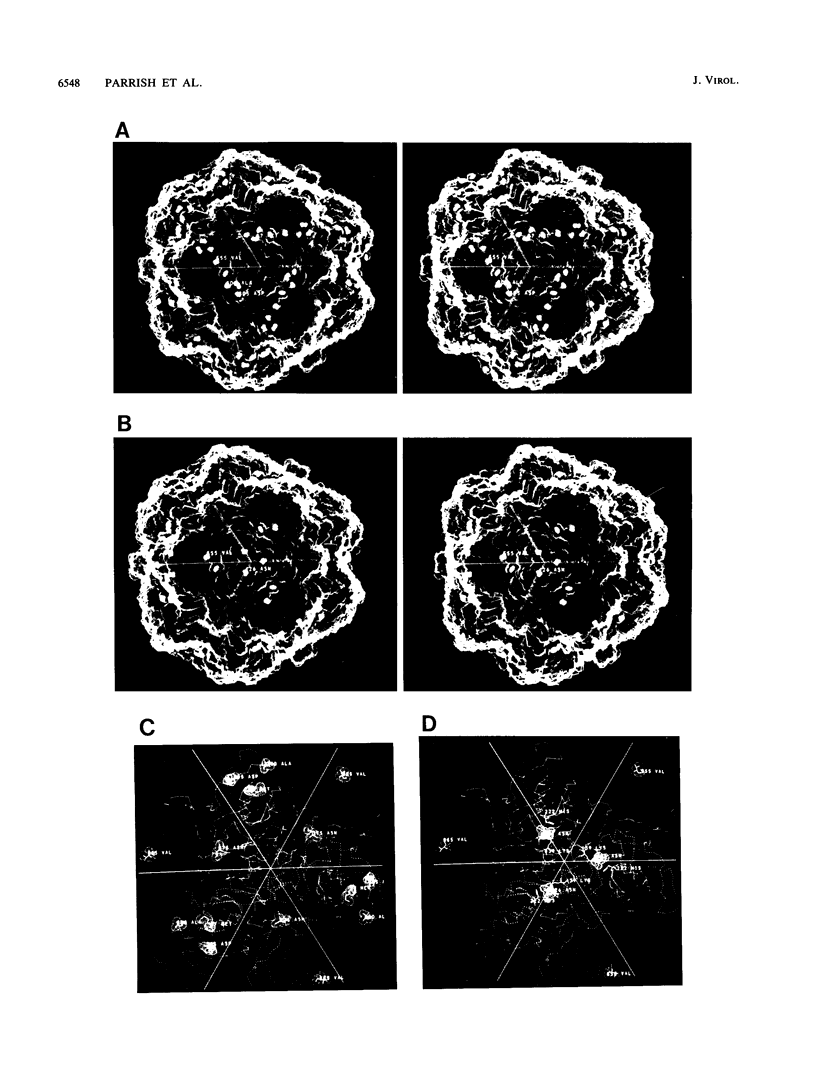



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Gibbs A. J., Laver W. G., Webster R. G. Evolutionary changes in influenza B are not primarily governed by antibody selection. Proc Natl Acad Sci U S A. 1990 May;87(10):3884–3888. doi: 10.1073/pnas.87.10.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfe P., Simmonds P., Ludlam C. A., Bishop J. O., Brown A. J. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J Virol. 1990 Dec;64(12):6221–6233. doi: 10.1128/jvi.64.12.6221-6233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I., Faragher S. G., Vrati S., Dalgarno L. Genetic stability of Ross River virus during epidemic spread in nonimmune humans. Virology. 1988 Dec;167(2):639–643. [PubMed] [Google Scholar]
- Burtonboy G., Coignoul F., Delferriere N., Pastoret P. P. Canine hemorrhagic enteritis: detection of viral particles by electron microscopy. Arch Virol. 1979;61(1-2):1–11. doi: 10.1007/BF01320586. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Crawford A. M., Zelazny B. Evolution in Oryctes baculovirus: rate and types of genomic change. Virology. 1990 Jan;174(1):294–298. doi: 10.1016/0042-6822(90)90078-6. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Leiter J. M., Li X. Q., Palese P. Positive Darwinian evolution in human influenza A viruses. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4270–4274. doi: 10.1073/pnas.88.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammelin M., Altmüller A., Reinhardt U., Mandler J., Harley V. R., Hudson P. J., Fitch W. M., Scholtissek C. Phylogenetic analysis of nucleoproteins suggests that human influenza A viruses emerged from a 19th-century avian ancestor. Mol Biol Evol. 1990 Mar;7(2):194–200. doi: 10.1093/oxfordjournals.molbev.a040594. [DOI] [PubMed] [Google Scholar]
- Gojobori T., Moriyama E. N., Kimura M. Molecular clock of viral evolution, and the neutral theory. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10015–10018. doi: 10.1073/pnas.87.24.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Fomsgaard A., Allan J., Gravell M., London W. T., Olmsted R. A., Hirsch V. M. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J Virol. 1990 Mar;64(3):1086–1092. doi: 10.1128/jvi.64.3.1086-1092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegae Y., Sugita S., Endo A., Ishida M., Senya S., Osako K., Nerome K., Oya A. Evolutionary pattern of the hemagglutinin gene of influenza B viruses isolated in Japan: cocirculating lineages in the same epidemic season. J Virol. 1990 Jun;64(6):2860–2865. doi: 10.1128/jvi.64.6.2860-2865.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Bean W. J., Webster R. G. Evolution of the hemagglutinin of equine H3 influenza viruses. Virology. 1989 Apr;169(2):283–292. doi: 10.1016/0042-6822(89)90153-0. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Krauss S., Webster R. G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989 Nov;63(11):4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koptopoulos G., Papadopoulos O., Papanastasopoulou M., Cornwell H. J. Presence of antibody cross-reacting with canine parvovirus in the sera of dogs from Greece. Vet Rec. 1986 Mar 22;118(12):332–333. doi: 10.1136/vr.118.12.332. [DOI] [PubMed] [Google Scholar]
- Luo L. Z., Li Y., Snyder R. M., Wagner R. R. Spontaneous mutations leading to antigenic variations in the glycoproteins of vesicular stomatitis virus field isolates. Virology. 1990 Jan;174(1):70–78. doi: 10.1016/0042-6822(90)90055-v. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Takeda N., Tanimura M., Ogino T., Yamazaki S., Chen C. W., Lin K. H., Lin S. Y., Ghafoor A., Yin-Murphy M. Evolutionary study on the Coxsackievirus A 24 variant causing acute hemorrhagic conjunctivitis by oligonucleotide mapping analysis of RNA genome. Arch Virol. 1990;114(1-2):37–51. doi: 10.1007/BF01311010. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Tanimura M., Takeda N., Kono R., Yamazaki S. Evolution of enterovirus 70 in nature: all isolates were recently derived from a common ancestor. Arch Virol. 1986;89(1-4):1–14. doi: 10.1007/BF01309875. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Konishi S., Ajiki M., Akaboshi T. Comparison of feline parvovirus subspecific strains using monoclonal antibodies against a feline panleukopenia virus. Nihon Juigaku Zasshi. 1989 Apr;51(2):264–272. doi: 10.1292/jvms1939.51.264. [DOI] [PubMed] [Google Scholar]
- Morinet F., Tratschin J. D., Perol Y., Siegl G. Comparison of 17 isolates of the human parvovirus B 19 by restriction enzyme analysis. Brief report. Arch Virol. 1986;90(1-2):165–172. doi: 10.1007/BF01314155. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Rowe J. E., Fitch W. M. Glycoprotein evolution of vesicular stomatitis virus New Jersey. Virology. 1989 Feb;168(2):281–291. doi: 10.1016/0042-6822(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Orito E., Mizokami M., Ina Y., Moriyama E. N., Kameshima N., Yamamoto M., Gojobori T. Host-independent evolution and a genetic classification of the hepadnavirus family based on nucleotide sequences. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7059–7062. doi: 10.1073/pnas.86.18.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Burtonboy G., Carmichael L. E. Characterization of a nonhemagglutinating mutant of canine parvovirus. Virology. 1988 Mar;163(1):230–232. doi: 10.1016/0042-6822(88)90255-3. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E., Antczak D. F. Antigenic relationships between canine parvovirus type 2, feline panleukopenia virus and mink enteritis virus using conventional antisera and monoclonal antibodies. Arch Virol. 1982;72(4):267–278. doi: 10.1007/BF01315223. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Antigenic structure and variation of canine parvovirus type-2, feline panleukopenia virus, and mink enteritis virus. Virology. 1983 Sep;129(2):401–414. doi: 10.1016/0042-6822(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986 Jan 15;148(1):121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Gorham J. R., Schwartz T. M., Carmichael L. E. Characterization of antigenic variation among mink enteritis virus isolates. Am J Vet Res. 1984 Dec;45(12):2591–2599. [PubMed] [Google Scholar]
- Parrish C. R., Have P., Foreyt W. J., Evermann J. F., Senda M., Carmichael L. E. The global spread and replacement of canine parvovirus strains. J Gen Virol. 1988 May;69(Pt 5):1111–1116. doi: 10.1099/0022-1317-69-5-1111. [DOI] [PubMed] [Google Scholar]
- Parrish C. R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991 Jul;183(1):195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., O'Connell P. H., Evermann J. F., Carmichael L. E. Natural variation of canine parvovirus. Science. 1985 Nov 29;230(4729):1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Nucleotide sequence of the coat protein gene of canine parvovirus. J Virol. 1985 May;54(2):630–633. doi: 10.1128/jvi.54.2.630-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. Polymorphism and evolution of influenza A virus genes. Mol Biol Evol. 1986 Jan;3(1):57–74. doi: 10.1093/oxfordjournals.molbev.a040381. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda M., Hirayama N., Itoh O., Yamamoto H. Canine parvovirus: strain difference in haemagglutination activity and antigenicity. J Gen Virol. 1988 Feb;69(Pt 2):349–354. doi: 10.1099/0022-1317-69-2-349. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Srinivasan A., Schochetman G., Marcus M., Myers G. The phylogenetic history of immunodeficiency viruses. Nature. 1988 Jun 9;333(6173):573–575. doi: 10.1038/333573a0. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Martinez M. A., Carrillo C., Beck E. Antigenic variation of foot-and-mouth disease virus of serotype C during propagation in the field is mainly restricted to only one structural protein (VP1). Virus Res. 1989 Dec;14(4):273–280. doi: 10.1016/0168-1702(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Umene K., Nunoue T. The genome type of human parvovirus B19 strains isolated in Japan during 1981 differs from types detected in 1986 to 1987: a correlation between genome type and prevalence. J Gen Virol. 1990 Apr;71(Pt 4):983–986. doi: 10.1099/0022-1317-71-4-983. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., de Jong J. J., Van den Berg H., Tijnagel J. M., Krone W. J., Goudsmit J. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9938–9942. doi: 10.1073/pnas.87.24.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]