Abstract
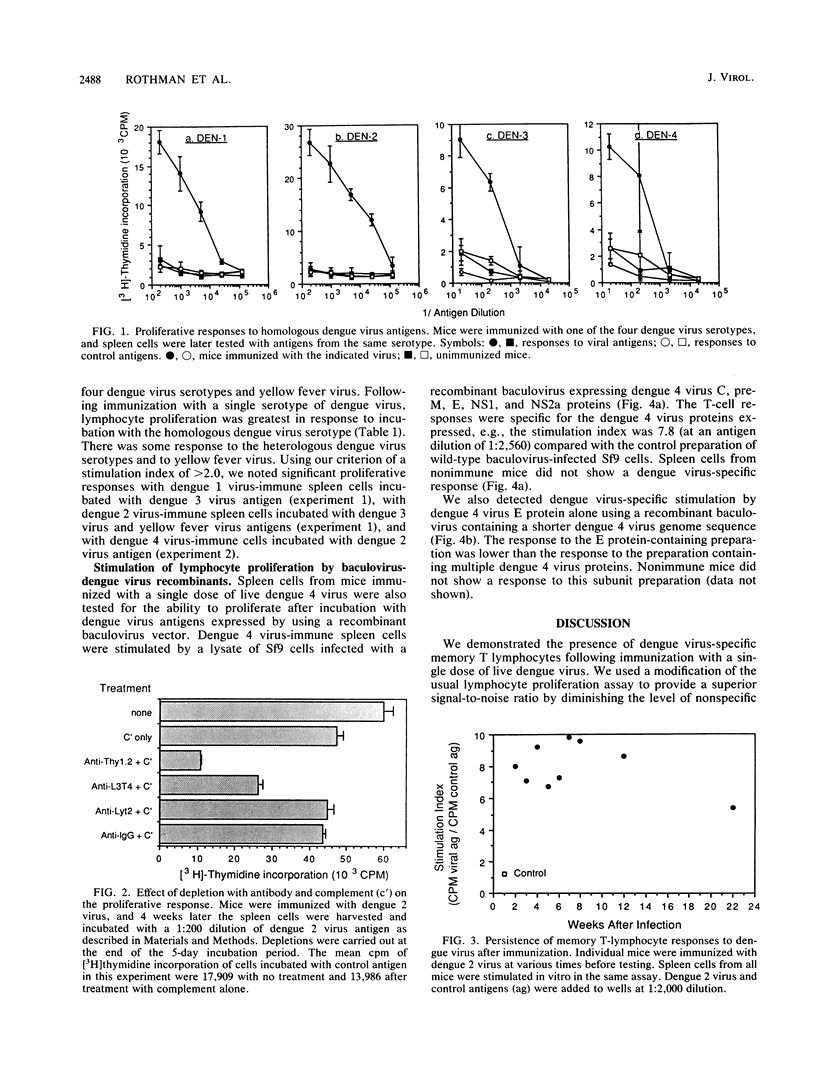
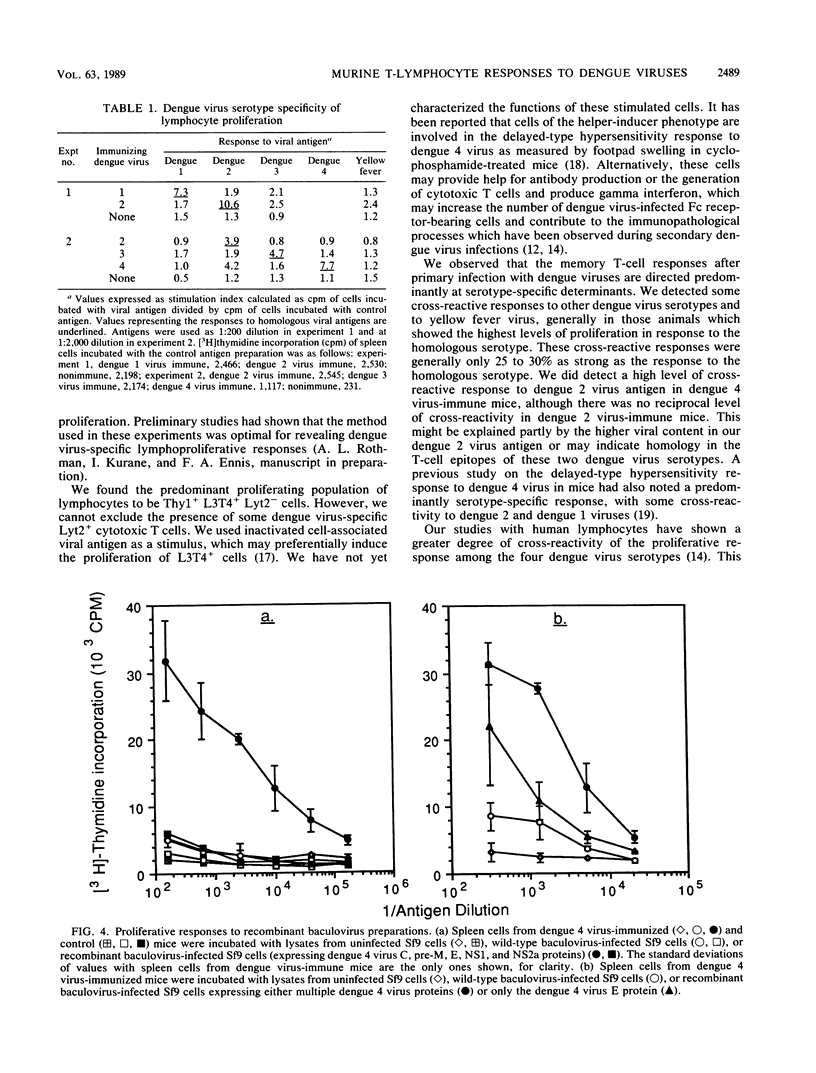
Definition of the T-lymphocyte responses to dengue viruses should aid in the development of safe and effective vaccines and help to explain the pathophysiology of dengue hemorrhagic fever and dengue shock syndrome. In this study, we demonstrated that dengue virus-specific T lymphocytes were detected in spleen cells from dengue virus-immune mice using an in vitro proliferation assay. Following immunization with a single dose of infectious dengue virus, murine lymphocytes showed increased proliferation when incubated in the presence of viral antigens of the same serotype but not in the presence of control antigens. Depletion experiments with antibody and complement showed that the population of responding cells expressed the Thy1+ L3T4+ Lyt2- phenotype. This indicates that the predominant proliferating cells are T lymphocytes of the helper-inducer phenotype. Dengue virus-specific memory lymphocyte responses were detectable for at least 22 weeks after immunization. The response to primary infection was primarily serotype specific, with some serotype cross-reactivity present at a low level. We demonstrated that lymphocytes from mice immunized with dengue 4 virus proliferate in response to a combination of dengue 4 virus C, pre-M, E, NS1, and NS2a proteins expressed in Sf9 cells with a recombinant baculovirus, and, to a lesser extent, to the dengue 4 virus E protein alone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandriss M. W., Schlesinger J. J., Walsh E. E., Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986 Feb;67(Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- Brandt W. E. From the World Health Organization. Current approaches to the development of dengue vaccines and related aspects of the molecular biology of flaviviruses. J Infect Dis. 1988 May;157(5):1105–1111. doi: 10.1093/infdis/157.5.1105. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Johnson D. E., Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988 Jan;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Chain B., McCafferty I., Wallace G., Askenase P. W. Improvement of the in vitro T cell proliferation assay by a modified method that separates the antigen recognition and IL-2-dependent steps. J Immunol Methods. 1987 May 20;99(2):221–228. doi: 10.1016/0022-1759(87)90131-1. [DOI] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Esposito J. J., Cropp C. B., Vorndam A. V., Monath T. P., Trent D. W. Dengue 2 virus envelope protein expressed by a recombinant vaccinia virus fails to protect monkeys against dengue. J Gen Virol. 1988 Aug;69(Pt 8):1921–1929. doi: 10.1099/0022-1317-69-8-1921. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. Dengue haemorrhagic fever--a public health problem and a field for research. Bull World Health Organ. 1980;58(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Cohen S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970 Apr;42(5):311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988 Jan 29;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Henchal E. A., McCown J. M., Burke D. S., Seguin M. C., Brandt W. E. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985 Jan;34(1):162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- Kaufman B. M., Summers P. L., Dubois D. R., Eckels K. H. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987 Mar;36(2):427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- Kontny U., Kurane I., Ennis F. A. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988 Nov;62(11):3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Innis B. L., Nisalak A., Hoke C., Nimmannitya S., Meager A., Ennis F. A. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Invest. 1989 Feb;83(2):506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E., Makino Y., Zhao B. T., Zhang Y. M., Markoff L., Buckler-White A., Guiler M., Chanock R., Lai C. J. The nucleotide sequence of dengue type 4 virus: analysis of genes coding for nonstructural proteins. Virology. 1987 Aug;159(2):217–228. doi: 10.1016/0042-6822(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Morens D. M., Halstead S. B. Disease severity-related antigenic differences in dengue 2 strains detected by dengue 4 monoclonal antibodies. J Med Virol. 1987 Jun;22(2):169–174. doi: 10.1002/jmv.1890220208. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T., Devi S., Yeen W. P., McKenzie I. F., Leong Y. K. Lyt phenotype and H-2 compatibility requirements of effector cells in the delayed-type hypersensitivity response to dengue virus infection. Infect Immun. 1984 Jan;43(1):429–431. doi: 10.1128/iai.43.1.429-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T., Wong P. Y., Pathmanathan R. Induction and characterization of delayed-type hypersensitivity to dengue virus in mice. J Infect Dis. 1982 Aug;146(2):235–242. doi: 10.1093/infdis/146.2.235. [DOI] [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S., Jatanasen S., Salitul V., Phanthumachinda B., Halstead S. B. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984 Nov;120(5):653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1987 Mar;68(Pt 3):853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- Uren M. F., Doherty P. C., Allan J. E. Flavivirus-specific murine L3T4+ T cell clones: induction, characterization and cross-reactivity. J Gen Virol. 1987 Oct;68(Pt 10):2655–2663. doi: 10.1099/0022-1317-68-10-2655. [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Hayes E. P., McCarty T. C., Dubois D. R., Summers P. L., Eckels K. H., Chanock R. M., Lai C. J. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induces resistance to dengue virus encephalitis. J Virol. 1988 Aug;62(8):3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



