Abstract
Interleukin 10 (IL-10) is a recently described natural endogenous immunosuppressive cytokine that has been identified in human, murine, and other organisms. Human IL-10 (hIL-10) has high homology with murine IL-10 (mIL-10) as well as with an Epstein–Barr virus genome product BCRFI. This viral IL-10 (vIL-10) shares a number of activities with hIL-10. IL-10 significantly affects chemokine biology, because human IL-10 inhibits chemokine production and is a specific chemotactic factor for CD8+ T cells. It suppresses the ability of CD4+ T cells, but not CD8+ T cells, to migrate in response to IL-8. A nonapeptide (IT9302) with complete homology to a sequence of hIL-10 located in the C-terminal portion (residues 152–160) of the cytokine was found to possess activities that mimic some of those of hIL-10. These are: (i) inhibition of IL-1β-induced IL-8 production by peripheral blood mononuclear cell, (ii) inhibition of spontaneous IL-8 production by cultured human monocytes, (iii) induction of IL-1 receptor antagonistic protein production by human monocytes, (iv) induction of chemotactic migration of CD8+ human T lymphocytes in vitro, (v) desensitization of human CD8+ T cells resulting in an unresponsiveness toward rhIL-10-induced chemotaxis, (vi) suppression of the chemotactic response of CD4+ T human lymphocytes toward IL-8, (vii) induction of IL-4 production by cultured normal human CD4+ T cells, (viii) down-regulation of tumor necrosis factor-α production by CD8+ T cells, and (ix) inhibition of class II major histocompatibility complex antigen expression on IFN-γ-stimulated human monocytes. Another nonapeptide (IT9403) close to the NH2-terminal part of hIL-10 did not reveal cytokine synthesis inhibitory properties, but proved to be a regulator of mast cell proliferation. In conclusion, we have identified two functional domains of IL-10 exerting different IL-10 like activities, an observation that suggests that relatively small segments of these signal proteins are responsible for particular biological functions.
Human interleukin-10 (IL-10), a recently described natural endogenous immunosuppressive cytokine, was originally identified because of its inhibitory effect on cytokine production (1, 2). Additionally, IL-10 counterparts have been identified in a number of other species including the mouse, rat, and pig. Human IL-10 suppresses the release and function of a number of proinflammatory cytokines such as IL-1, tumor necrosis factor-α (TNF-α), and IL-6, thereby representing a normal endogenous feedback factor when it comes to the control of immune responses and inflammation. Apart from modulating an array of cytokine functions, human IL-10 (hIL-10) also is a chemotactic factor toward CD8+ T cells, whereas it suppresses the ability of CD4+, but not CD8+, T cells to migrate in response to the T cell chemotactic cytokine IL-8 (4). The suppressive effect of IL-10 on chemotaxis moreover is achieved through the direct inhibitory effect that IL-10 exerts on chemokines, such as the production of IL-8. Through suppressing the production of IL-8, which also is an inhibitor of IL-4 production, IL-10 indirectly contributes to a TH2-like immune response (11). Further, human and viral IL-10 are able to inhibit antigen-specific T cell proliferation by modulating the antigen-presenting capacity of monocytes through down-regulation of the class II major histocompatibility complex (MHC) antigen expression (8). In addition to this, human IL-10 has also been demonstrated to affect the growth of leukocytes because it was observed to induce apoptosis in cultured B-Leukemia cells (5).
IL-10 is relatively species-specific, but, for example, the viral IL-10 and the human IL-10 share a number of biological functions, such as the capacity to deactivate human as well as murine macrophages (6, 9). Murine, human, and viral IL-10 inhibit cytokine synthesis by monocytes and TH1 cells (1, 3), but the murine IL-10, in turn, appears to be inactive on human cells (1, 4). Another example of species differences is that the viral IL-10, in contrast to murine and human IL-10, does not up-regulate class II MHC antigen expression on murine splenic B lymphocytes (7).
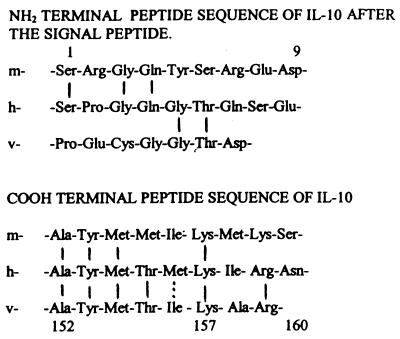
There is 71% homology between the murine and the human DNA encoding mature IL-10 proteins, whereas the viral IL-10 shows 84% homology with the human IL-10 with respect to the amino acid sequence (1). Supported by the fact that murine IL-10 does not appear active on human cells, including a lack of chemotactic activity toward human CD8+ T cells (4), and that viral and human IL-10 both act on human cells, we explore parts of the IL-10 amino acid chain, where the viral and the human IL-10 appeared to exhibit higher homology to each other than to their murine counterpart (Fig. 1).
Figure 1.
Predicted amino and carboxyl acid terminal sequences of murine IL-10, human IL-10, and viral IL-10 (2).
At the carboxyl-terminal end of human IL-10, a part of the peptide counting 9 aa (Ala-Tyr-Met-Thr-Met-Lys-Ile-Arg-Asn) is characterized by the fact that 6 aa are homologous to the viral IL-10, whereas only 4 aa are homologous to the amino acids found in the same region of the murine IL-10. We synthetized an oligopeptide (IT9302) that is homologous to this 9-aa long part of the C-terminal end of the human IL-10 and tested it for possible IL-10 functions. During this process we also synthetized a number of other nonapeptides that all were homologous to different domains of the mature hIL-10. Among those, we tested nonapeptides representing the NH2-terminal portion of hIL-10. These sequences were chosen because of their low degree (less than 33%) of homology between the human and viral counterparts of IL-10, with respect to these particular domains.
MATERIALS AND METHODS
Synthetic Oligopeptides.
The chemical synthesis of IT9302 was carried out by using an automatic polypeptide synthesizer performed by Carlbiotech (Copenhagen) and Schafer-N (Copenhagen). The purity of the protein was confirmed to exceed 95% by HPLC, and the molecular weight was controlled by mass spectroscopy. IT9302 includes the amino acid sequences Ala-Tyr-Met-Thr-Met-Lys-Ile-Arg-Asn. Additionally, a nonapeptide identical to the NH2-terminal part of hIL-10, containing Ser-Pro-Gly-Gln-Gly-Thr-Gln-Ser-Glu (residues 1–9), was synthetized. Likewise a nonapeptide, identical to a portion of hIL-10 close to the NH2-terminal end (Ser-Glu-Asn-Ser-Cys-Thr-His-Phe-Pro) (residues 8–16), was synthesized and named IT9403. In addition, a number of other nonapeptides with amino acid sequence identity to human IL-10 but less than 33% identity to viral were synthesized.
Cytokines and Chemoattractants.
Recombinant hIL-10 was obtained from PeproTech, Boston (catalog no. 200 10), and rhIL-10 was also obtained from R & D Systems (catalog no. 217-IL-005). Recombinant hIL-1β and recombinant hIL-8 were kind gifts from Dianippon Pharmaceutical, Osaka. The culture medium was RPMI 1640 (GIBCO, cat. no. 6187–010) lipopolysaccharide (LPS)-free, according to the Limulus Amoebocyte Lysate assay (Sigma E-Toxate Kit, cat. no. 210-A1).
Production of IL-8 by Normal Human Peripheral Blood Mononuclear Cells (PBMCs).
PBMCs were purified from heparinized blood of normal human donors. After gradient centrifugation with Lymphoprep (Nycomed, Oslo), the mononuclear cells were diluted to 2 × 106 cells per ml in RPMI 1640 medium (GIBCO, catalog no. 6187–010) containing 1% sterile filtered heat-inactivated fetal calf serum and penicillin (10,000 units/ml), streptomycin (10 mg/ml), and gentamycin (2.5 mg/ml). Cells were cultured in 24 wells of Nunc Micro Plates and in the presence of different concentrations of IT9302 (1000, 100, … 0.01, and 0 ng/ml) for 24 hr. After 24 hr of incubation, an additional dose of IT9302 was added, and 1 hr later rhIL-1β (1 ng/ml) was added to the cell cultures. Supernatants were collected after a total of 48 hr of incubation, and the concentration of the secreted IL-8 was measured by using an IL-8 ELISA Kit (Dianippon Pharmaceutical, Osaka).
Spontaneous Production of IL-8 by Purified Monocytes.
PBMCs were purified as described above, and the monocytes were isolated by a plastic adherence technique and washed with Hanks’ solution, with 1% FCS at 23°C. Monocytes were then cultured in RPMI 1640 with 2% FCS including penicillin, streptomycin, and gentamycin at a cell concentration of 3 × 106/ml. Cells were cultured for 40 hr in microwells (Nunc) together either with 100 ng/ml rhIL-10 or IT9302 (1000, 100, … 0.01, and 0 ng/ml). The supernatants were collected, and the secreted IL-8 was measured.
Determination of TNF-α Production by CD8+ T Lymphocytes.
CD8+ T lymphocytes were purified from heparinized normal human blood (4) and cultured at a concentration of 2.5 × 106 cells/ml in RPMI 1640 containing 1% sterile-filtrated fetal calf serum (including penicillin, streptomycin, and gentamycin). The cells were cultured for 3 days with rhIL-8 (100 ng/ml) alone or with IT9302 (10, 1, and 0 ng/ml) added 30 min before rhIL-8. T cells and culture media were separated by centrifugation, and cells were resuspended directly in 100 μl of gel lysis buffer (10) and were kept at −80°C until further examined. The TNF-α content in the cytosolic fraction was evaluated by blotting proteins from one-dimensional 15% SDS/PAGE gels (11) onto Hybond-ECL nitrocellulose membranes (Amersham, RPN 2020D). The blots were blocked and incubated with a polyclonal rabbit anti-human TNF-α antibody (500-P31A, PeproTech) and a horseradish peroxidase-labeled secondary antibody (catalog no. P 217, Dako). This was followed by enhanced chemiluminescence activation (catalog no. RPN 2106, ECL, Amersham), and the immunostaining was detected by exposing a film (Kodak X-Omat-S) for 30 sec. The intensity of immunostaining was determined by densitometry by using quant image software (Molecular Dynamics).
Determination of IL-1 Receptor Antagonistic Protein (IRAP) Concentration.
PBMCs were purified as described above. The monocytes were purified by a plastic adherence technique and cultured 2.5 × 106 cells per ml in RPMI 1640 with 2% FCS in the presence of rhIL-10 or IT9302. The cells were stimulated for 24 hr, and the supernatants were collected for IRAP determination. In some experiments, mAb for IL-10 sR (R & D Systems, catalog no. MAB 274) was also added 1 hr before rIL-10. IRAP was determined by using an IRAP ELISA (human IL-1ra quantikine, Immunoassay Kit from R & D Systems (cat. no. DRA 00).
T Cell Chemotaxis.
CD4+ and CD8+ T lymphocytes were purified from heparinized blood of normal donors (4). The chemotaxis assay is a 48-well microchamber technique (Neuroprobe, Cabin John, MD) as previously described (4). In one experiment, the chemotactic activity of IT9302 on CD8+ T cells was performed by testing serial dilution of IT9302 added to the lower chamber and evaluating chemotaxis as described above. In a second experiment, the ability of IT9302 to desensitize the migration of CD8+ T cells as a response to rhIL-10 (10 ng/ml) was studied by adding IT9302 to the target cells 30 min before chemotaxis. Thus, IT9302 was added in serial concentrations, and the chemotactic response to rhIL-10 in the lower chamber was evaluated as described above. In a third experiment, the ability of IT9302 to suppress the chemotactic response of CD4+ T cells toward rh-IL-8 (10 ng/ml) was studied by adding IT9302 to the target cells 30 min before performing chemotaxis. IT9302 was added in serial concentrations and the chemotactic response to rh-IL-8 in the lower chamber was evaluated as described above.
MC/9 Proliferation Assay.
The biological activity of IT9302 and IT9403 was determined by using a proliferation assay as described by Thompson-Snipes et al. (16). Cells from a murine mast cell line, MC/9 (25 × 103 cells per ml), were cultured in DMEM (GIBCO, cat. no. 61965–026) containing 10% FCS, 6 μg/ml folic acid, 50 μM 2-mercaptoethanol + 1 mM sodium pyruvate + 0.1 mM non-essential amino acids (GIBCO, cat. no. 11140–035). IT9302, IT9403, or IL-10 was added (in equimolar concentrations to IL-10): 2 ng/ml or 20 ng/ml in combination with rIL-4 (20 ng/ml) (PeproTech), rIL-10 (R & D Systems, cat. no. 217-IL-005). Further, cells cultured with higher doses of IT9302, IT9403, or IL-10 were added 2 μg/ml of IL-10 sR monoclonal antibody (R & D Systems, MAB 274) 1 hr before adding IT9302, IT9403, or IL-10. MC/9 cells were stimulated for 3 days, and the number of cells was estimated by counting (16).
HLA-DR Expression on Human Monocytes.
The monocytes were isolated from fresh, heparinized blood by adherence to plastic at 37°C in RPMI 1640 containing 10% FCS. After incubation the supernatant was removed and Hanks’ solution (4°C with 1% FCS) was added. The cells were detached by cooling at −20°C for 15 min and then gently vibrating the culture plate. The monocytes were then cultured (2 × 106 cells per ml) in RPMI 1640 with 2% FCS and stimulated for 40 hr with IFN-γ (10 ng/ml), and then either rhIL-10 (100, 10, 1, and 0 ng/ml) added 1 hr before IFN-γ or IT9302 (10, 1, 0.1, and 0 ng/ml) or IT9403 (10, 1, 0.1, and 0 ng/ml) added 1 hr before IFN-γ for 40 hr. The cells were detached from the wells by cooling as mentioned above. The cells were resuspended in Hanks’ solution with 1% FCS, 1 × 106 cells per ml, and FITC-conjugated mouse-anti-human antibody recognizing HLA-DR, DQ, and DR antigen was added (F 0817, Dako) for 45 min. The cells were washed three times in Hanks’ solution, and a FACS analysis was performed by using a Coulter–Epics XL-MCL flow cytometer at a wavelength of 488 nm. Nonspecific binding was determined by using a nonrelevant FITC-conjugated antibody (mouse anti-goat, Dako F 479).
Alternatively, the cells after stimulation, as described above, were fixed in 10% DMSO, 40% RPMI 1640, and 50% sterile FCS and stored at −80°C until DNA was typed as described below (12).
DNA Typing.
The fixed cells were suspended in 70% ethanol for 60 min and then washed twice in Hanks’ solution. Cells (1 × 106) were resuspended in 250 μl RNase (1 μg/ml) (Pharmacia, 17-0442-01) in 1.12% sodium-citrate, pH 8.4, and kept at 37°C for 3 min. Thereafter, 250 μl propidium iodide (50 μg/ml in Hanks’ solution) was added, and the cells were kept without light for 30 min. Subsequently, the cells were washed twice in Hanks’ solution, a technique optimized by Jensen et al. (12). The DNA content was measured by flow cytometry on a Coulter–Epics XL-MCL at a wavelength of 550 nm. The fraction of cells expressing DNA in G1 or G2 phase of cell proliferation as well as the fraction expressing apoptosis were determined. The fraction of apoptotic cells can be estimated by extraction of the degraded low-molecular-weight DNA from permeabilized cells followed by staining with a DNA fluorochrome propidium iodide. The lower DNA content of the apoptotic cells is visualized by weaker fluorochrome staining. Thus, cells exhibiting lower DNA staining than that of G1 cells, the so-called sub-G1 peak, are considered apoptotic.
Immunization of Rabbits.
Keyhole limpet hemocyanin (KLH) (Sigma, H-7017) was coupled to IT9302 by Schafer-N. IT9302 (250 μg) coupled to KLH was injected subcutaneously as an emulsion in complete subsequently incomplete Freund’s adjuvant (Sigma F-5881, respectively Sigma F-5506). The injections were repeated four times, with 2-week intervals, and the rabbits were bled 8 to 12 days thereafter. The formation of antibody was tested by Western blotting.
Specificity of the IT9302 Antibody.
Induction of IRAP by monocytes was achieved by adding 10 ng/ml IT9302 or 100 ng/ml rhIL-10 (see above). The specificity of a polyclonal antibody for IT9302 was tested by adding 2 μl rabbit serum to 1 ml monocyte cell culture medium 1 hr before IT9302 or rhIL-10 was added. The rabbit serum was also purified from antibodies for the fusion protein (KLH) by absorption of the serum to KLH-coated Maxisorp plates (Life Technologies, Roskilde, Denmark, cat. no. 00442404) at 4°C overnight. The IRAP content in the supernatants was measured by using a Human IL-1ra Quantikine Immunoassay Kit (R & D Systems).
The specificity of the antibody was also tested by Western Blotting for rhIL-10. Normal nonimmune rabbit serum was used as negative control in both IRAP induction experiments and Western blotting.
RESULTS
Biophysical Characterization of IT9302.
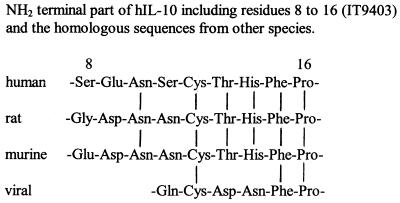
IT9302 has a calculated molecular mass of 1,127 Da, compared with the molecular mass of rhIL-10, which is 18,400 Da. According to this, 6.125 ng/ml of IT9302 approximately equals 100 ng/ml of IL-10 in molarity. Amino acid sequences of IT9302 was described in Fig. 1 and Materials and Methods. IT9403 has a calculated molecular mass of 1,020 Da, and the amino acid sequences of the peptide were described in Fig. 7 and Methods.
Figure 7.
The amino acid sequence of IT9403 residues 8–16 from hIL-10 and the homologous sequences from rat, murine, and viral IL-10.
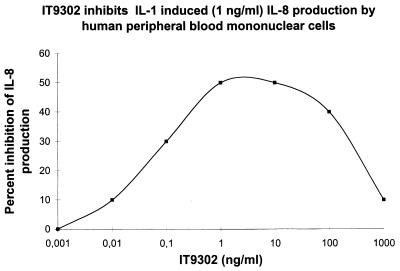
IT9302 Inhibits IL-1β-Induced IL-8 Production by Human Peripheral Blood Mononuclear Cells.
Human peripheral blood mononuclear cells were stimulated as described in Materials and Methods. As shown in Fig. 2, IT9302 inhibited the IL-1β-induced production of IL-8 by human peripheral blood mononuclear cells in a dose-dependent manner. The inhibition of IL-8 production plateaus between 1 and 10 ng/ml of IT9302, where the IL-8 production was suppressed to 50%. A nonapeptide at the NH2-terminal part of hIL-10, residues 1–9, did not inhibit the IL-1β-induced production of IL-8 by human peripheral blood mononuclear cells, and this peptide sequence does not have the quality of significant sequence homology to the viral IL-10 as is the case of IT9302 (see Fig. 1).
Figure 2.
IL-8 secretion by PBMCs cultured for 24 hr with IT9302 (0, 0.001, … , 1,000 ng/ml). A second dose of IT9302 was added after 23 hr; 1 hr later rhIL-1β (1 ng/ml) was added for a further 24 hr. The supernatants were collected and the IL-8 content was measured by using an IL-8 ELISA assay. The figures shows the result from a representative experiment of three experiments.
IT9302 Inhibits Spontaneous IL-8 Production by Human Monocytes.
Monocytes were purified and stimulated as described in the Materials and Methods. IT9302 inhibited the spontaneous IL-8 production by monocytes. Thus, at 1 ng/ml of IT9302, the IL-8 production was suppressed to a level of 35% of the spontaneous production. One hundred nanograms per milliliter of IL-10 inhibited IL-8 production to a similar level (data not shown).
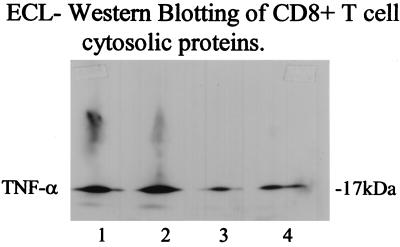
TNF-α Production by CD8+T Lymphocytes.
Purified CD8+ T cells were cultured and the cells were collected and analyzed for TNF-α content in the cytosolic fraction by using Western blotting. As seen in Fig. 3, 10 ng/ml of IT9302 down-regulated the TNF-α production significantly (more than 50%) after 3 days in cell culture.
Figure 3.
TNF-α production by CD8+ human T lymphocytes was measured after culturing the cells for 3 days. Lanes: 1, control; 2, rhIl-8 (100 ng/ml) added alone; 3, IT9302 (10 ng/ml); and 4, IT9302 (1 ng/ml) added 30 min before rhIL-8. The cells were collected, and 100 μl gel lysis buffer was added. The TNF-α content was determined by Western blotting.
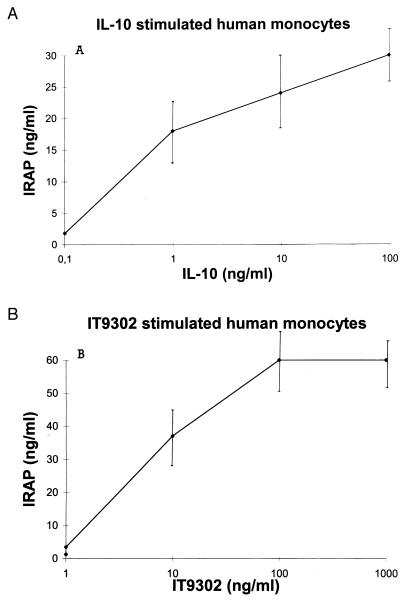
IT9302 Induces IRAP Production by Human Monocytes.
Monocytes were stimulated with either IT9302 or IL-10. We observed that 100 ng/ml of rhIL-10 up-regulated the IRAP production to 30 ng/ml. Also, 10 ng/ml IT9302 induced IRAP production to a level of 35 ng/ml in the same time period of 24 hr (Fig. 4). This means that the specific activities of IT9302 and rhIL-10 are comparable and approximately equal with respect to induction of IRAP. A monoclonal antibody for IL-10 sR (2 μg/ml) was unable to block IL-10 (100 ng/ml) as well as IT9302 (10 ng/ml)-induced IRAP production (data not shown), whereas the induction of IRAP production by IT9302 or IL-10 was effectively neutralized by a polyclonal rabbit IT9302 antibody (see below).
Figure 4.
IRAP production by IL-10- (A) or IT9302 (B)-stimulated human monocytes (24 hr). IRAP was measured by using an IRAP ELISA assay.
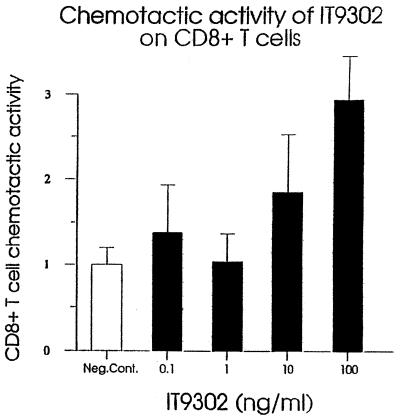
IT9302 Is Chemotactic to Human CD8+ T Lymphocytes.
As shown in Fig. 5, IT9302 induced the chemotactic migration of CD8+ human T lymphocytes, whereas there was no effect on CD4+ T cells (data not shown). Again, the potency of IT9302 observed in this experiment is comparable with the activity of recombinant hIL-10, as shown previously (figure 1 in ref. 4).
Figure 5.
Chemotactic activity of IT9302 on CD8+ human T cells.
IT9302 Desensitizes Human CD8+ T Cells Resulting in an Unresponsiveness Toward rhIL-10.
IT9302 was added to a suspension of CD8+ T cells 30 min before the cells were tested for their chemotactic response to rhIL-10. Preincubation of cells with IT9302 results in a suppressed responsiveness of the CD8+ T cells to rhIL-10. This indicates that IT9302 may affect the binding of rhIL-10 to an IL-10 receptor (data not shown).
IT9302 Suppressed the Chemotactic Response of CD4+ T Lymphocytes Toward IL-8.
The experiment was carried out as described above. IT9302, added to a suspension of human CD4+ T lymphocytes, inhibits the response of CD4+ T cells toward IL-8 in a dose-dependent manner. Thus, by using 10 ng/ml or more of IT9302 suppressed the chemotactic response to the level of spontanous migration (data not shown).
Regulation of Murine Mast Cell MC/9 Proliferation by IT9302 and IT9303.
Murine mast cells were stimulated as described in the Methods by using equimolar amounts of IT9302, IT9403, or IL-10 combined with IL-4. The cells cultured with the highest doses of IT9302, IT9403, or IL-10 were also cultured in the presence of a mAb for IL-10 sR, added 1 hr before the IT9302, IT9403, or IL-10 (Table 1). Comparing IT9302 and IT9403, cells were more activated by IT9403, and this stimulation could be blocked significantly (52.1%) by adding 2 μg/ml of the mAb for the IL-10 sR (Table 1).
Table 1.
The effect of IL-10 and its synthetic analogues, IT9302 and IT9403, on the growth/proliferation of murine mast cells (MC/9), when cultured in the presence of the costimulatory factor IL-4.
| Cytokine/oligopeptide | A Total number of cells in culture | B Total number of cells in cultures pretreated with IL-10sR-mAb for 1 hr | (A-B)/A Relative reduction (%) of the number of cells in cultures as result of IL-10 sR pretreatment |
|---|---|---|---|
| IL-4, 20 ng/ml | 47,000 ± 2,000 | ||
| IL-4 + IL-10 | |||
| (2 ng/ml) (2 ng/ml) | 48,000 ±0 | ||
| (20 ng/ml) (20 ng/ml) | 66,000 ± 6,000 | 52,000 ± 4,000 | 21.2 |
| IL-4 + IT9302 | |||
| (20 ng/ml) (2 ng/ml) | 33,000 ± 1,000 | ||
| (20 ng/ml) (20 ng/ml) | 40,000 ± 0 | 31,000 ± 1,000 | 21.9 |
| IL-4 + IT9403 | |||
| (20 ng/ml) (2 ng/ml) | 48,000 ± 1,000 | ||
| (20 ng/ml) (20 ng/ml) | 64,000 ± 8,000 | 31,000 ± 4,000 | 52.1 |
| IL-4 + IT9403 + IT9302 | |||
| (20 ng/ml) (2 ng/ml) (2 ng/ml) | 45,000 ± 1,000 | ||
| (20 ng/ml) (20 ng/ml) (20 ng/ml) | 79,000 ± 25,000 | 36,000 ± 14,000 | 54.3 |
In some of the cultures, the cells were pretreated for 1 h with a monclonal antibody toward IL-10 sR (2 μg/ml).
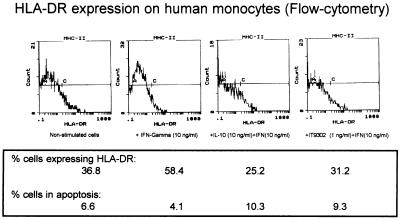
HLA-DR Expression on Human Monocytes.
The monocytes were purified as described above and cultured (2 × 106 cells per ml) in medium for 40 hr in the presence of IFN-γ (10 ng/ml) alone or together with rhIL-10, IT9302, or IT9403 added 30 min before IFN-γ. The MHC II antigen expression was studied by FACS analysis by using FITC-conjugated mouse-anti-human antibody for HLA-DR, DP, DK antigen. The percentage of cells expressing MHC II antigen was up-regulated by IFN-γ from 36.8 to 58.4% (see Fig. 6), whereas rhIL-10 (10 ng/ml) or IT9302 (1 ng/ml) added 30 min before IFN-γ prevented the induction of HLA-DR expression and actually down-regulated the MHC II antigen expression to 25.2 and 31.2%, respectively (Fig. 6). Tested in the same experiment, 1 ng/ml of IT9403 added 30 min before IFN-γ also prevented the induction of the MHC II antigen expression to the level of 33.7% (data not shown).
Figure 6.
HLA-DR expression on human monocytes (flow cytometry) regulated by IFN-γ (10 ng/ml) alone or IL-10 (10 ng/ml) or IT9302 (1 ng/ml) added 1 hr before IFN-γ. In the same fraction the DNA content was measured as described in Materials and Methods, and the fraction of cells in apoptosis was calculated.
DNA Typing of the Stimulated Monocytes.
IFN-γ-stimulated monocytes (1 × 106 cells per ml) obtained from the experiment mentioned above were incubated with propidium iodide, and the DNA content in the cells was measured by flow cytometry. As seen in Fig. 6, nonstimulated monocytes were expressing apoptosis in 6.6% of the cells. IFN-γ stimulation reduced apoptosis to 4.1% whereas both IL10 + IFN-γ and IT9302 + IFN-γ stimulation of the cells increased the apoptotic fraction to 10.3% and 9.3%, respectively (Fig. 6). IT9403 + IFN-γ stimulation did not induce apoptosis. Thus, 3.4% of the cells were expressing apoptosis, which is less than the level achieved by IFN-γ stimulation (data not shown).
The Specificity of the IT9302 Antibody.
Purified monocytes were cultured with 10 ng/ml IT9302 or 100 ng/ml rhIL-10 for 24 hr, as described above. Serum (2 μl) from IT9302-immunized rabbits was tested by adding it to 1 ml of monocyte cell culture. The IRAP secretion from the unstimulated cells was 3.5 ng/ml and from the stimulated cells was 10.6 ± 0.6 ng/ml, and this level of IRAP was suppressed to 2.9 ± 0.3 ng/ml when 2 μl IT9302 AB serum was added 1 hr before IT9302 to the monocyte culture. In another experiment, IT9302 (10 ng/ml) and rIL-10 (100 ng/ml) induced IRAP to a level of 37 ng/ml and 46 ng/ml, respectively, and these could be suppressed by 2 μl purified IT9302 AB serum to 27.5 ng/ml and 25.0 ng/ml IRAP, respectively. Serum from nonimmunized rabbits did not block IRAP induction. In contrast, a rat monoclonal hIL-10 antibody (JES3–19F1) was unable to affect the IRAP induction by IT9302, whereas JES3–19F1 was able to neutralize the effect of endogenous IL-10 in LPS-stimulated monocytes (3).
DISCUSSION
Although the complex structure of proteins (such as cytokines) as a whole determines features that are responsible for the biological fate of these potent signal molecules, such as their distribution, target specificity, and ultimately elimination from the organism, it has been demonstrated in recent years that occasionally smaller molecules such as oligopeptides or other low-molecular-weight nonpeptide structures also may act as functional agonists for the intact protein with respect to binding and signaling through the cellular cytokine receptors. In the present article, we have studied whether the biological functions of the immunosuppressive cytokine IL-10 in part can be confined to particular segments of the mature cytokine, IL-10. Based on our knowledge of differences and similarities among human, murine, and viral IL-10 with respect to species specificity, we identified domains of the mature protein that we suspected to be of importance for the receptor binding and signaling of IL-10. To test our hypothesis, we synthesized oligopeptides with complete homology to selected sequences of the human IL-10 and observed that important IL-10-like activities are determined by a part of the C-terminal portion of human IL-10 because a synthetic counterpart, i.e., a nonapeptide (IT9302), exhibited several IL-10-like biological in vitro activities. In particular, we found that IT9302, like IL-10 (2, 4, 8), possesses cytokine synthesis inhibitory effects, thus suppressing IL-1β-stimulated IL-8 production by monocytes. IT9302 also affected proinflammatory cytokine activities through inducing the production of the IRAP by monocytes, a feature adding to the immunosuppressive properties of IT9302 as well as the IL-10 domain, which it represents. Thereby, IT9302 generally may inhibit a variety of proinflammatory functions for which IL-1 is responsible (13, 14).
A critical step for inflammation is the migration of leukocytes to the site of the inflammatory reaction events, which are orchestrated through focal synthesis and release of chemokines. We observed also that the response of target cells, in this case CD4+ T cells, to chemokines such as IL-8 is regulated by IT9302 as well as the mature cytokine hIL-10, because both of these inhibited the migration of CD4+ T cells as a response to IL-8. Additionally, both IT9302 (Fig. 5) and IL-10 (4) are chemotactic toward CD8+ T cells. These observations suggest that the same domain on IL-10, namely the C-terminal portion, determines the cytokine synthesis inhibitory activity and the effect of IL-10 on cellular migration.
During the course of cell-mediated immune reactions, such as allergic type IV reactions or rejection of transplanted organs, the involvement of class II antigens is pivotal, and IL-10 plays a significant role in the regulation of the expression of the class II antigens. Hence, IL-10 inhibits IFN-γ-induced class II antigen expression on mononuclear cells (8), a function that appears to be confined to the segment of IL-10 represented by IT9302. Based on these observations, we find that some of the functions most important for the regulation of immune-response and inflammation are defined by the part of the IL-10 molecule that is homologous to IT9302.
During the study of IL-10 functions and, in particular, domains of IL-10, we have synthesized a number of oligopeptide homologous to various parts of the mature IL-10 molecule and screened these candidates for IL-10-like activity. One of these oligopeptides, a nonapeptide called IT9403, was identical to a segment of IL-10 (residues 8–16) and did not exhibit cytokine synthesis inhibitory activities, nor did it induce apoptosis, but it did exhibit regulation of class II antigen expression. Also, IT9403, in contrast to IT9302, induced the proliferation of murine mast cells, a function that it shares with IL-10, and this function could be inhibited by the addition of a monoclonal antibody toward the recently identified soluble IL-10 receptor (17). This supports indirectly the hypothesis that these synthetic analogues may actually bind to the IL-10 receptor(s), and it suggests that particular functions of the IL-10 molecule are defined by certain domains of protein structure. Because the IT9403 segment appears in many species (see Fig. 7) but is only represented by three amino acids in the viral IL-10, it can explain that the vIL-10 lacks activity on murine cells, no enhancement of class II MHC expression on murine B cells (7), and no costimulatory effect on murine mast cell proliferation (2, 26). Other investigators have shown that the viral IL-10 does not bind to the IL-10 sR (18). This supports the hypothesis that IT9403 is essential for binding to the IL-10 sR.
An important normal biological feature of IL-10 is its restraining effect on the consequences of endotoxin or LPS on the living organism, because IL-10 inhibits the production and consequences of highly potent proinflammatory cytokines, in particular, TNF-α, a cytokine that plays a crucial role for the development of the sepsis syndrome. The importance of IL-10 in this context has been demonstrated through experiments that showed the capacity of IL-10 to protect mice from lethal endotoxemia (24). Interestingly, neither IT9302 nor IT9403 affected LPS-induced production of cytokines (TNF-α and IL-6) in vitro, whereas we observed that when we combined these two nonapeptides we obtained an approximately 30% inhibition of TNF-α and IL-6 production. In contrast, equimolar amounts of IL-10 resulted in almost complete suppression (>95%) of the production of these cytokines (data not shown). Consequently, the regulatory functions of IL-10 on LPS-induced inflammation cannot be confined to the same segment (LPS) that appears to regulate cytokine production in a broad manner. It is possible that this explained that the cytokine cascade induced by LPS is mediated through several pathways, an assumption supported by the observations of Dinarello et al. (25) that IL-1β is not essential for the LPS-induced production of cytokines such as TNF-α and IL-6. The question that arises as a consequence of these observations is whether mediators operating through the same cellular receptors may exhibit differences in signal profiles, such as is the case for IL-10 and IT9302. This appears possible, with references to the fact that such diversities in signal transduction already are known. Thus, IL-8 and GRO-α, two closely related chemokines operating through identical receptors, reveal significant differences in their induction of phospholipase D (Teizo Yoshimura, personal communication).
The IL-10 receptor is a member of the class-2 cytokine receptor family that includes IFN-γRα, a soluble cellular surface receptor, and IFN-γRβ1, which acts as a species-specific accessory factor 1 or subunit (19–21). Analysis of the crystal structure of the receptor complex between IFN-γ and its receptors reveals a probable binding domain at the N-terminal part at the so-called AB loop of IFN-γ. However, the deletion of the C-terminal IFN-γ residues 122–138 revealed a significant drop in IFN-γ receptor binding (22). Based on the topological similarity of IL-10 and IFN-γ, Zdanov et al. (23) created a model of an IL-10 dimer interacting with two soluble receptors. This showed that the contact region between the cytokine and the receptors is predicted to be based on a complementary polar and hydrophobic interaction. The model implicates that the interface between IL-10 and its receptor also includes the C-terminal portion of IL-10, where the possible hydrogen bonds and ionic interaction in the interface included residues Asp-144, Asn-148, Glu-151, Arg-159, and Asn-160 (23).
Thus, this analysis supports the possibility that residues 152–160 at the C-terminal end of IL-10, homologous to IT9302, are capable of interacting with the IL-10 receptor(s).
To evaluate whether IT9302 also exhibits IL-10-like activities in vivo we recently tested the biological effects of this nonapeptide in a model of severe acute necrotic pancreatitis and found that pretreatment of rabbits with IT9302 significantly reduced the serum levels of TNF-α and IL-8 as well as reduced the mortality of the rabbits (M. O. Osmann, S. B. Lansten, N. O. Jacobsen, J. U. Kristensen, B. Deleuran, M. Deleuran, B.G., C.G.L., and S.L.J., unpublished data).
In conclusion, we found that the biological functions of a cytokine, in this case IL-10, may be confined to particular and relatively small-sized segments of the mature cytokine. Our data also suggest that different domains of the mature cytokine define different properties. Hence, we found that a synthetic counterpart of the C-terminal portion of IL-10, the nonapeptide IT9302, exerted a variety of biological properties to those of IL-10. That we recently found IT9302 to exhibit significant IL-10-like activity in vivo encourages further research in the biological properties and pharmaceutical potential of the segment of IL-10 that is identical to IT9302.
Acknowledgments
This research was supported by a research grant from Nycomed Dak A/S, Copenhagen.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IL, interleukin; IRAP, IL-1 receptor antagonistic protein; MHC, major histocompatibility complex; TNF, tumor necrosis factor; LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell.
Data deposition: The sequence of IT9302 and IT9403 reported in this paper was deposited in the Swiss-Prot database (P22301).
References
- 1.Di-Hwei H, de Waal Malefyt R, Fiorentino D F, Minh-Ngoc Dang, Viera P, de Vries J, Spits H, Mosmann T M, Moore K M. Science. 1990;250:830–833. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 2.Viera P, de Waal Malefyt R, Dang M N, Johnson K E, Kastelein R, Fiorentino D F, de Vries J E, Roncarolo M-G, Mosmann T R, Moore K W. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Waal Malefyt R, Abrams J, Bennet B, Figdor C G, de Vries J. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jinquan T, Larsen C G, Gesser B, Matsushima K, Thestrup-Pedersen K. J Immunol. 1993;151:4545–4551. [PubMed] [Google Scholar]
- 5.Fluckinger A-C, Durand I, Banchereau J. J Exp Med. 1994;179:91–94. doi: 10.1084/jem.179.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore K W, O’Garra A, de Waal Malefyt R, Viera P, Mosmann T R. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 7.Go N F, Castle E, Barret R, Kastelein R, Dang W, Mosmann T R, Moore K W, Howard M. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M-G, te Velde A, Figdor C, Jonson K, Kastelein R, Yssel H, de Vries J E. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt R, Yssel H, Roncarolo M-G, Spits H, de Vries J E. Curr Opin Immunol. 1992;4:314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 10.Celis J E, Ratz G P, Madsen P, Gesser B, Lauritsen J B, Brogaard-Hansen K P, Kwee S, Rasmussen H H, Nielsen H, Cruger D, Basse B, Leffers H, Honore H, Møller O, Celis A. Electrophoresis. 1989;10:76–115. doi: 10.1002/elps.1150100204. [DOI] [PubMed] [Google Scholar]
- 11.Gesser B, Lund M, Lohse N, Vestergaard C, Matsushima K, Sindet-Pedersen S, Lindkær Jensen S, Thestrup-Pedersen K, Grønhøj Larsen C. J Leuk Biol. 1996;59:407–411. doi: 10.1002/jlb.59.3.407. [DOI] [PubMed] [Google Scholar]
- 12.Jensen I M, Kristensen J S, Thomsen M, Ellegaard J, Høkland P. Anal Cell Pathol. 1993;5:213–223. [PubMed] [Google Scholar]
- 13.McIntyre K, Stepan G J, Kolinsky K D, Benjamin W R, Plocinski J M, Kaffka K L, Campen C A, Chizzonite R A, Kilian P L. J Exp Med. 1991;173:931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli G, Kinter A L, Fauci A S. Proc Natl Acad Sci USA. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T R, Moore K W. Immunol Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 16.Thompson-Snipes L, Dhar V, Bond M W, Mosman T R, Moore K W, Rennick D M. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan J C, Braun S, Rong H, DiGiacomo R, Dolphin E, Baldwin S, Narula S K, Zavodny P J, Chou C C. J Biol Chem. 1995;270:12906–12911. doi: 10.1074/jbc.270.21.12906. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, de Waal Malefyt R, Briere F, Parham C, Bridon J M, Banchereau J, Moore K W, Xu J. J Immunol. 1997;158:604–613. [PubMed] [Google Scholar]
- 19.Walter M R, Windsor W T, Nagabhushan T L, Lundell D J, Lunn C A, Zauodny P J, Narula S K. Nature (London) 1995;376:230–235. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- 20.Soh J, Donelly R J, Kotenko S, Marino T M, Cook J R, Wang N, Emanuel S, Swarts B, Miki T, Pestka S. Cell. 1994;76:793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi S, Bohni R, Stark G, Di Marco F, Aguet M. Cell. 1994;76:803–810. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 22.Griggs N D, Jarpe M A, Jarpe M A, Pace J L, Russel S W, Johson H M. J Immunol. 1992;149:517–520. [PubMed] [Google Scholar]
- 23.Zdanov A, Schalk-Hihi C, Wlodawer A. Protein Sci. 1996;5:1955–1962. doi: 10.1002/pro.5560051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard M, Muchamuel T, Andrade S, Menon S. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe J D, Shaw A L, Dinarello C A. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 26.MacNeil I, Suda T, Moore K W, Mosmann T R, Zlotnik A. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]