Abstract
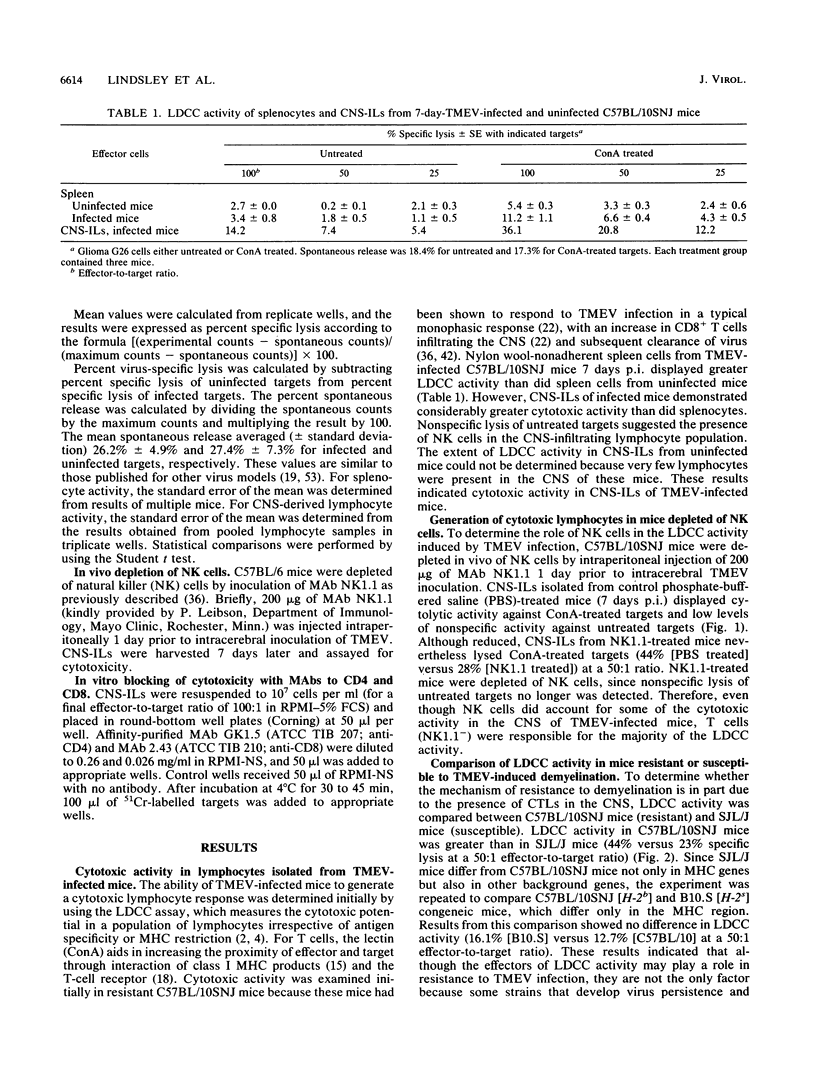
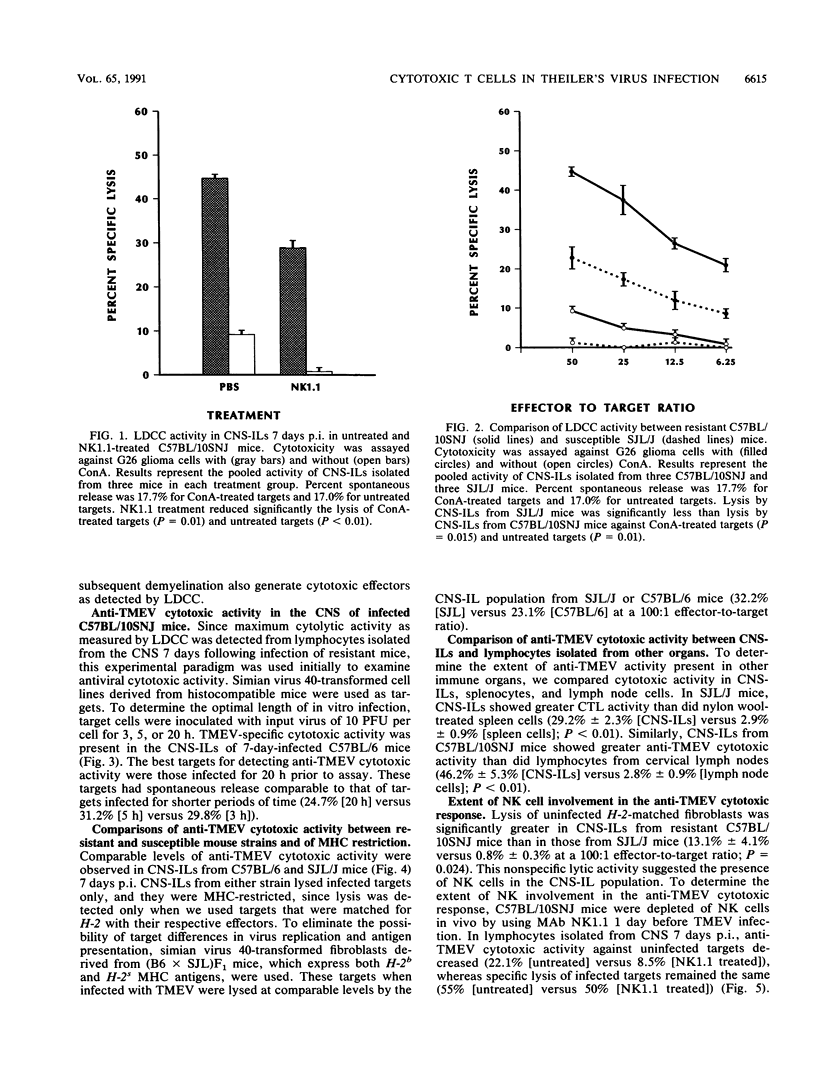
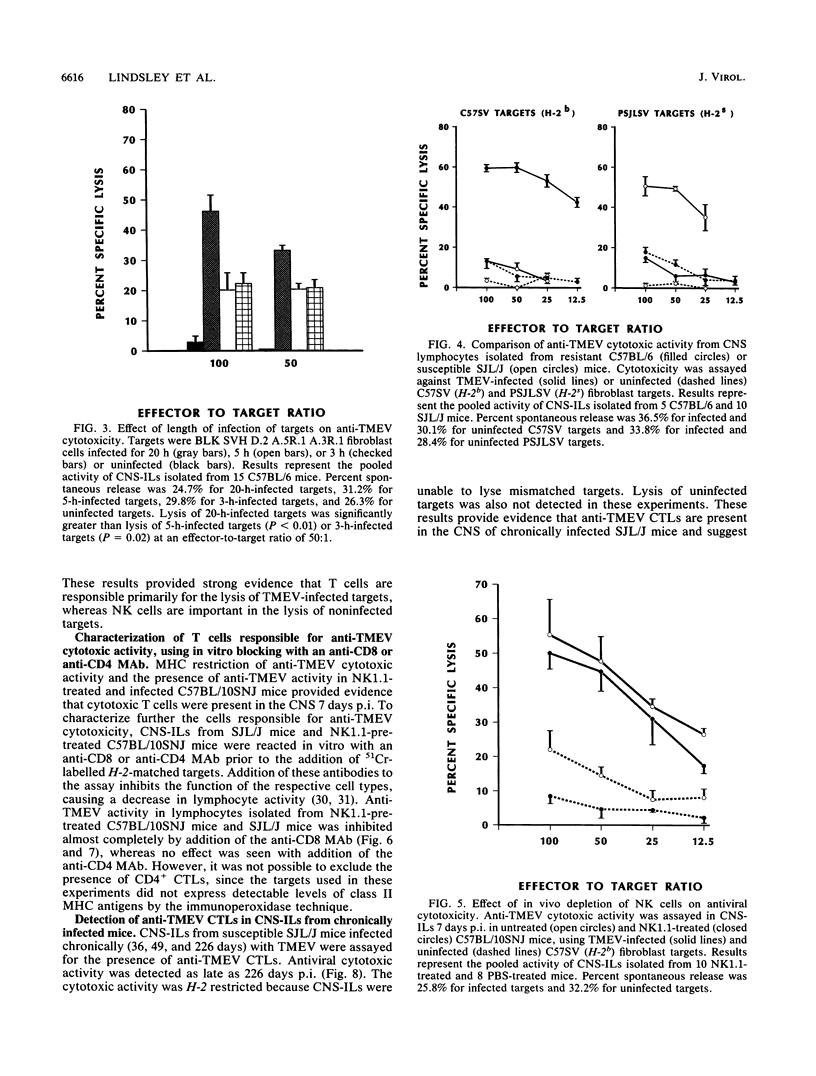
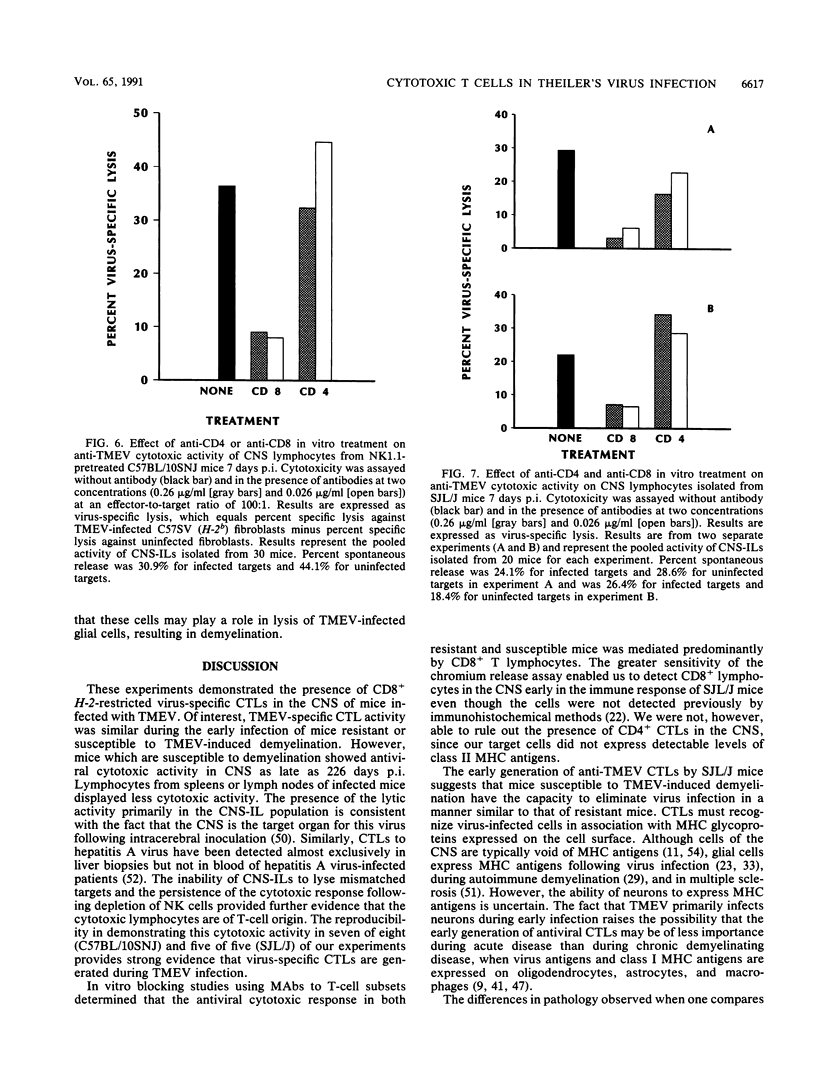
Intracerebral inoculation of resistant mice (C57BL/10SNJ) with Theiler's murine encephalomyelitis virus (TMEV) results in acute encephalitis followed by subsequent clearance of virus from the central nervous system (CNS). In contrast, infection of susceptible mice (SJL/J) results in virus persistence and chronic immune-mediated demyelination. Both resistance and susceptibility to TMEV-induced disease appear to be immune mediated, since immunosuppression results in enhanced encephalitis in resistant mice but diminished demyelination in susceptible mice. The purpose of these experiments was to determine whether anti-TMEV cytotoxic T lymphocytes (CTLs) are generated during acute and chronic TMEV infection. Nonspecific lectin-dependent cellular cytotoxicity was used initially to detect the cytolytic potential of lymphocytes infiltrating the CNS irrespective of antigen specificity. Using TMEV-infected targets, H-2-restricted TMEV-specific CTLs of the CD8+ phenotype were demonstrated in lymphocytes from the CNS of susceptible and resistant mice, arguing against the hypothesis that the ability to generate CD8+ CTLs mediates resistance. In chronically infected SJL/J mice, TMEV-specific CTL activity was detected in the CNS as late as 226 days postinfection. These experiments demonstrate that virus-specific CTLs are present in the CNS during both acute and chronic TMEV infection. Anti-TMEV CTLs in the CNS of chronically infected SJL/J mice may play a role in demyelination through their ability to lyse TMEV-infected glial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Ferluga J., Janossy G. Non-specific cytotoxicity by T cells activated with plant mitogens in vitro and the requirement for plant agents during the killing reaction. Clin Exp Immunol. 1973 Dec;15(4):573–589. [PMC free article] [PubMed] [Google Scholar]
- Baenziger J., Hengartner H., Zinkernagel R. M., Cole G. A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986 Apr;16(4):387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- Bevan M. J., Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol. 1975 Feb;114(2 Pt 1):559–565. [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- DANIELS J. B., PAPPENHEIMER A. M., RICHARDSON S. Observations on encephalomyelitis of mice (DA strain). J Exp Med. 1952 Dec;96(6):517–530. doi: 10.1084/jem.96.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Ultrastructural immunohistochemical localization of virus in acute and chronic demyelinating Theiler's virus infection. Am J Pathol. 1982 Jan;106(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- Date I., Kawamura K., Nakashima H. Histological signs of immune reactions against allogeneic solid fetal neural grafts in the mouse cerebellum depend on the MHC locus. Exp Brain Res. 1988;73(1):15–22. doi: 10.1007/BF00279656. [DOI] [PubMed] [Google Scholar]
- Dixon J. E., Allan J. E., Doherty P. C. The acute inflammatory process in murine lymphocytic choriomeningitis is dependent on Lyt-2+ immune T cells. Cell Immunol. 1987 Jun;107(1):8–14. doi: 10.1016/0008-8749(87)90260-7. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Gorman K. C., Kane K. P., Clark W. R. Target cell recognition structures in LDCC and ODCC. J Immunol. 1987 Feb 15;138(4):1014–1019. [PubMed] [Google Scholar]
- Hubbard S. C., Kranz D. M., Longmore G. D., Sitkovsky M. V., Eisen H. N. Glycosylation of the T-cell antigen-specific receptor and its potential role in lectin-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1852–1856. doi: 10.1073/pnas.83.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. In vitro culture of coxsackievirus group B, type 3 immune spleen cells on infected endothelial cells and biological activity of the cultured cells in vivo. Infect Immun. 1984 Feb;43(2):567–573. doi: 10.1128/iai.43.2.567-573.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Knobler R. L., Lublin F. D., Hart M. N. Differential modulation of MHC class I antigen expression on mouse brain endothelial cells by MHV-4 infection. J Neuroimmunol. 1989 May;22(3):241–253. doi: 10.1016/0165-5728(89)90022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky S. G., Milisauskas V., Chen P. B., Nakamura I. Defective differentiation of natural killer cells in SJL mice. Role of the thymus. J Immunol. 1987 Feb 15;138(4):1020–1025. [PubMed] [Google Scholar]
- Lindsley M. D., Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989 Apr 15;142(8):2677–2682. [PubMed] [Google Scholar]
- Lipton H. L., Canto C. D. Contrasting effects of immunosuppression on Theiler's virus infection in mice. Infect Immun. 1977 Mar;15(3):903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Long E. O., Jacobson S. Pathways of viral antigen processing and presentation to CTL: defined by the mode of virus entry? Immunol Today. 1989 Feb;10(2):45–48. doi: 10.1016/0167-5699(89)90303-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. In situ detection of class I and II major histocompatibility complex antigens in the rat central nervous system during experimental allergic encephalomyelitis. An immunohistochemical study. J Neuroimmunol. 1986 Oct;12(4):265–277. doi: 10.1016/0165-5728(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Hodgdon J. C., Hercend T., Schlossman S. F., Reinherz E. L. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982 Oct 29;218(4571):471–473. doi: 10.1126/science.6981845. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Salvato M., Tishon A., Lewicki H. Virus-lymphocyte interactions. III. Biologic parameters of a virus variant that fails to generate CTL and establishes persistent infection in immunocompetent hosts. Virology. 1988 Jun;164(2):507–516. doi: 10.1016/0042-6822(88)90565-x. [DOI] [PubMed] [Google Scholar]
- Olsson T., Maehlen J., Löve A., Klareskog L., Norrby E., Kristensson K. Induction of class I and class II transplantation antigens in rat brain during fatal and non-fatal measles virus infection. J Neuroimmunol. 1987 Oct;16(2):215–224. doi: 10.1016/0165-5728(87)90076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Illavia S. J., Webb H. E. The identification and role of cells involved in CNS demyelination in mice after Semliki Forest virus infection: an ultrastructural study. Prog Brain Res. 1983;59:237–254. doi: 10.1016/S0079-6123(08)63869-8. [DOI] [PubMed] [Google Scholar]
- Patick A. K., Pease L. R., David C. S., Rodriguez M. Major histocompatibility complex-conferred resistance to Theiler's virus-induced demyelinating disease is inherited as a dominant trait in B10 congenic mice. J Virol. 1990 Nov;64(11):5570–5576. doi: 10.1128/jvi.64.11.5570-5576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya C. V., Patick A. K., Leibson P. J., Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J Immunol. 1989 Jul 1;143(1):95–102. [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., David C. S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985 Sep;135(3):2145–2148. [PubMed] [Google Scholar]
- Rodriguez M., Lafuse W. P., Leibowitz J., David C. S. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology. 1986 Jul;36(7):964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Lampert P. W. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983 Apr;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J., David C. S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986 Mar 1;163(3):620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Patick A. K., Pease L. R. Abrogation of resistance to Theiler's virus-induced demyelination in C57BL mice by total body irradiation. J Neuroimmunol. 1990 Mar;26(3):189–199. doi: 10.1016/0165-5728(90)90001-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Quddus J. Effect of cyclosporin A, silica quartz dust, and protease inhibitors on virus-induced demyelination. J Neuroimmunol. 1986 Dec;13(2):159–174. doi: 10.1016/0165-5728(86)90062-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Roos R. P., Wollmann R. DA strain of Theiler's murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984 May;15(5):494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Fujinami R. S., Lampert P. W. Mechanism of Theiler's virus-induced demyelination in nude mice. Lab Invest. 1986 May;54(5):515–522. [PubMed] [Google Scholar]
- Sethi P., Lipton H. L. Location and distribution of virus antigen in the central nervous system of mice persistently infected with Theiler's virus. Br J Exp Pathol. 1983 Feb;64(1):57–65. [PMC free article] [PubMed] [Google Scholar]
- Stallcup K. C., Springer T. A., Mescher M. F. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J Immunol. 1981 Sep;127(3):923–930. [PubMed] [Google Scholar]
- Vallbracht A., Maier K., Stierhof Y. D., Wiedmann K. H., Flehmig B., Fleischer B. Liver-derived cytotoxic T cells in hepatitis A virus infection. J Infect Dis. 1989 Aug;160(2):209–217. doi: 10.1093/infdis/160.2.209. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]


