Abstract
Activation of the recently identified c-Jun N-terminal kinases (JNKs) typically results in programmed cell death (apoptosis) in neurons and other cell types grown in culture. However, the effects of JNK activation in the central nervous system in vivo are unknown. At baseline, JNK activity in mice was on average 17-fold higher in brain than in peripheral organs, whereas JNK protein levels were similar. In brain, JNK was expressed primarily in neurons. Restraining mice or allowing them to explore a novel environment rapidly increased JNK activity 3- to 15-fold in various brain regions, but these manipulations did not increase brain activity of the extracellular signal-regulated kinase. Because noninvasive environmental stimuli that do not induce neurodegeneration elicited prominent increases in JNK activity in the brain, we conclude that acute activation of the JNK cascade in central nervous system neurons does not induce neuronal apoptosis in vivo. In contrast, the high baseline activity of JNK in the brain and the activation of the JNK cascade by environmental stimuli suggest that this kinase may play an important physiological role in neuronal function.
Eukaryotic cells respond to external stimuli via signal transduction pathways, many of which include protein Ser/Thr kinases of the mitogen-activated protein (MAP) kinase family. MAP kinases have been well conserved during evolution, suggesting that they fulfill important biological functions (1). The MAP kinase family contains at least two functionally distinct cascades: the extracellular signal-regulated kinase (ERK) cascade, which is activated by growth factors and appears to be responsible for cell growth and proliferation (2–4), and the recently characterized c-Jun N-terminal kinase (JNK) cascade, also called the stress-activated protein kinase cascade (5, 6). Thus far, 10 JNK isoforms with molecular masses of 46 kDa (JNK-46) or 55 kDa (JNK-55) have been cloned from human brain (7). The two groups of JNK isoforms are encoded by three genes designated JNK1, 2, and 3 (7). In cell culture (in vitro), the JNK cascade can be activated by a variety of genotoxic or environmental stresses (e.g., alkylating reagents, UV, ionizing radiation, and osmotic stress) (5, 8–11) or by inflammatory cytokines (e.g., tumor necrosis factor α and interleukin 1) (9, 12, 13). In most cases, in vitro activation of the JNK cascade primarily inhibits cell growth or induces cell death (13–15). For example, withdrawal of nerve growth factor from differentiated PC12 cells (rat adrenal pheochromocytoma) results in JNK activation and apoptosis (15). However, activation of the JNK cascade also has been associated with cell differentiation (16), cell proliferation (17), and tumorigenesis (18, 19). Biological consequences of JNK activation may depend on cell types and could differ under in vitro and in vivo conditions. Here, we report on the dynamic regulation of neuronal JNK activity in vivo.
MATERIALS AND METHODS
Animals, Cells, and Experimental Manipulations.
Two-month-old male C57BL/6 × SJL mice (The Jackson Laboratory) were used in this study. Unless indicated otherwise, mice were not manipulated experimentally (unmanipulated) until they were killed. Two methods were used to alter central nervous system (CNS) activity noninvasively: physical restraint (20) and exploration of a novel environment. For the restraint studies, mice were placed individually into a restrainer (Baxter Scientific Products, McGaw Park, IL) for 5 or 10 min or for 5 min followed by a 2-hr recovery period. For exploration of a novel environment, mice (housed two per cage) were transferred from their home cage (375 cm2) into a new cage (924 cm2) containing a bridge, a curved tunnel, and a hollow 14-sided geometric with one entrance hole on each side. Control mice were set down into the new cage for only 2–3 sec and returned immediately to their home cage for a 10-min observation period, whereas experimental mice were allowed to explore the new environment for 10 min. When mice were placed into the new cage individually during pilot experiments, they appeared anxious (freezing of movements, hiding) and were reluctant to explore the cage. In contrast, pairs of mice placed into the new cage together showed no evidence of anxiety and immediately began to explore the new environment. Consequently, for these exploration experiments, mice were placed into the new cage two at a time. At the end of the experiment, mice were killed by cervical dislocation. Organs were removed, dissected rapidly on ice, and snap-frozen. Snap-frozen tissues were either homogenized for enzyme assays and Western blot analyses or sectioned for immunohistochemistry. Neuro-2a cells (mouse neuroblastoma line, ATCC, CCL-131) were maintained in DMEM containing 10% fetal bovine serum and 5% horse serum. For differentiation, the DMEM was replaced with differentiation media (neurobasal media containing N-2 supplement) (GIBCO/BRL) for 48 hr.
Kinase Assays (18).
Solid-phase kinase assay. Fifty micrograms of tissue or whole-cell lysates were mixed with 10 μl of glutathione S-transferase (GST)-agarose resin suspension (Sigma) to which 5 μg of GST-c-Jun (amino acids 1–79) was bound. The mixture was agitated at 4°C for 4 hr and pelleted by centrifugation followed by washes in PBS/0.05% Triton X-100. The pelleted beads were suspended in 25 μl of kinase buffer (20 mM Hepes, pH 7.6/20 mM MgCl2/20 mM β-glycerolphosphate/20 mM p-nitrophenyl phosphate/0.1 mM Na3VO4/2 mM DTT) containing 10 μM ATP and 5 μCi of [γ32P] ATP. After 20 min at 30°C, the reaction was terminated by washing with PBS/0.05% Triton X-100. Phosphorylated proteins were eluted in 30 ml of Laemmli sample buffer and resolved on a SDS-polyacrylamide gel, followed by autoradiography or PhosphorImager (Fujix BAS 1000) analysis (21).
Immune complex kinase assay.
Proteins binding nonspecifically to tissue lysates first were removed by incubation with rabbit IgG-protein A agarose beads. ERK was precipitated with an antibody (prebound to protein A agarose beads) that specifically recognizes ERK1 and ERK2 (Santa Cruz Biotechnology). After a 4-hr incubation at 4°C, the ERK antigen-antibody bead complex was washed four times in PBS/0.05% Triton X-100 and pelleted by centrifugation. Kinase reactions were performed in 25 μl of kinase buffer containing 0.33 mg/ml of myelin basic protein at 30°C for 20 min. Phosphorylated myelin basic protein then was resolved on a 15% SDS-polyacrylamide gel, followed by autoradiography or PhosphorImager (Fujix BAS1000) analysis (21).
Western Blot Analysis.
Ten micrograms of tissue or whole-cell lysates were resolved on 10% SDS-polyacrylamide gels, blotted onto Immobilon transfer membranes (Millipore), and subjected to Western blot analysis by using an antibody that recognizes both JNK1 and JNK2, or an antibody that recognizes ERK1 and ERK2 (both antibodies from Santa Cruz Biotechnology). The antigen-antibody complexes were visualized with the ECL detection system (Amersham).
Immunohistochemistry.
Brain cryosections were postfixed with cold (−20°C) 95% ethanol for 15 min and blocked for 30 min each with 3% H2O2 and with sera from animals in which secondary antibodies were raised. Sections then were incubated overnight at 4°C with JNK antibodies: polyclonal antibody (Santa Cruz Biotechnology) or mAbs (JNK McAb 338.1 and JNK McAb 431; both were raised against recombinant JNK1 protein and specifically recognize JNK1 and JNK2; unpublished data). Primary antibody binding was detected after four washes in PBS by incubation of sections with species-matched biotinylated secondary antibodies for 1 hr at room temperature. Immunolabeling was revealed by immunoperoxidase reaction with ABC reagents (Vector Laboratories) and diaminobenzidine/H2O2 for development. Sections were imaged by light microscopy. As a specificity control, the polyclonal JNK antibody (1 μg) was adsorbed with 10 μg purified bacteria expressed-recombinant JNK1 overnight at 4°C before use in immunostaining as described. For staining of Neuro-2a cells, cells were fixed with 50% ethanol and 50% methanol for 30 min followed by sequential incubation with a JNK antibody (Santa Cruz Biotechnology) and goat anti-rabbit IgG-fluorescein isothiocyanate (Vector Laboratories). Immunolabeled cells were imaged by confocal microscopy (MRC1024, Bio-Rad).
RESULTS
JNK Activity at Baseline in the CNS and Peripheral Organs.
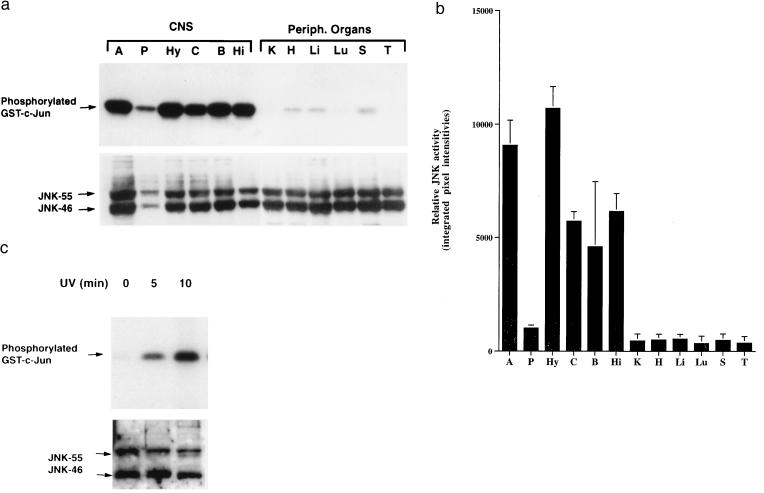
To elucidate potential roles of the JNK cascade in the CNS, we first examined JNK enzymatic activity and protein expression at baseline in various regions of the mouse brain (neocortex, hippocampus, brainstem, amygdala, hypothalamus, and pituitary) and in peripheral organs (liver, heart, lung, kidney, spleen, and thymus). Because all identified JNK isoforms bind to c-Jun by interacting with its δ-domain (5), we used a solid-phase kinase assay with glutathione S-transferase-c-Jun as the substrate to obtain overall JNK enzymatic activity in whole-tissue lysates. JNK activity was 10- to 24-fold higher in the neocortex, hippocampus, brainstem, hypothalamus, and amygdala than in peripheral organs (Fig. 1 a and b).
Figure 1.
High levels of JNK activity in the CNS. (a) Representative autoradiograph demonstrating substantially higher basal levels of JNK activity (Upper) in different brain regions [amygdala (A), pituitary (P), hypothalamus (Hy), neocortex (C), brainstem (B), and hippocampus (Hi)] than in peripheral organs [kidney (K), heart (H), liver (Li), lung (Lu), spleen (S), and thymus (T)]. JNK protein levels (Lower) in brain regions and peripheral organs revealed by Western blot analysis. (b) Semiquantitative comparison of JNK activities in CNS regions and peripheral organs. Signals from gels such as the one shown in a were quantitated by PhosphorImager analysis essentially as described (21). Each bar represents the mean JNK activity (error bars SD) identified in three mice in the same kinase assay. Similar results were obtained in two additional independent kinase assays. (c) JNK activation in Neuro-2a cells irradiated with UV-C for 0, 5, or 10 min. Whole-cell lysates were used for JNK enzymatic assay (Upper) and Western blot analysis (Lower).
JNK transcripts have been detected in mammalian brains at levels similar to those in peripheral organs (7). To determine if the high JNK activity in the CNS is caused by high levels of JNK protein expression, JNK immunoreactivity in tissue lysates was determined by semiquantitative Western blot analysis with an antibody specific for JNKs. Protein levels of JNK-46 and JNK-55 in most regions of the CNS were similar to those in peripheral organs (Fig. 1a, Lower), suggesting that the high levels of JNK activity in the CNS are not caused by increased JNK expression. In cultured Neuro-2a cells, baseline JNK protein levels were similar to those in the CNS, whereas baseline JNK activity was much lower (Fig. 1c). UV-C irradiation of these neuronal cultures up-modulated JNK activity to levels comparable to those found in the CNS at baseline (Fig. 1), and the elevated JNK activity in the Neuro-2a cells was associated with apoptosis (data not shown).
Distribution of JNKs in the CNS.
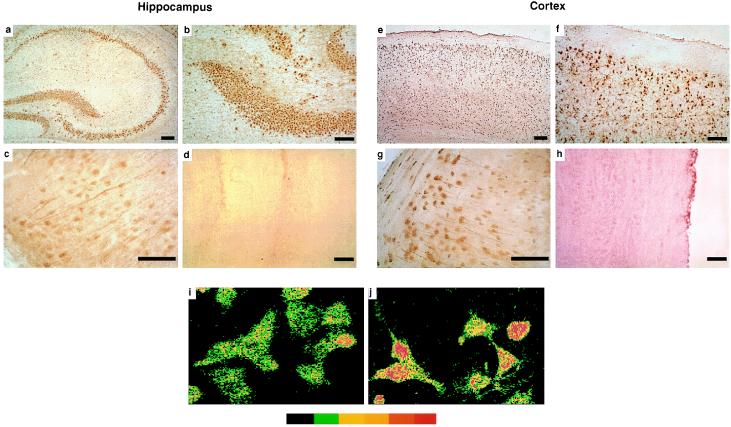
To determine which cell types express JNK in the CNS, mouse brain sections were immunostained with three different anti-JNK antibodies. All three antibodies revealed a similar pattern of JNK immunoreactivity (Fig. 2 and data not shown). Consistent with our Western blot analysis (Fig. 1a, Lower), immunostaining was widespread throughout the mouse brain. It was primarily cell-associated, and most immunolabeled cells were identified as neurons based on location and morphology (Fig. 2 a–c and e–g). These findings are consistent with results obtained in a previous study by in situ hybridization (22). JNK immunoreactivity was seen in neuronal cell bodies and in neuronal processes (Fig. 2 c and g). Some neurons showed stronger nuclear JNK immunostaining than others (Fig. 2g), suggesting JNK nuclear translocation. To determine whether increased nuclear JNK immunoreactivity may correspond to JNK activation, UV-C irradiated and untreated Neuro-2a cells were immunostained with JNK antibody, and replicate cultures were subjected to solid-phase kinase assay and Western blot analysis. JNK activation in Neuro-2a cells was associated with nuclear translocation of JNK immunoreactivity (Fig. 2 i and j). This is consistent with previous studies showing an association of JNK activation with nuclear translocation in mouse fibroblasts (23).
Figure 2.
Neuronal expression of JNK immunoreactivity in brain and cell culture. (a–h) Cryosections (10 μm) of hippocampus (a–d) and neocortex (e–h) of unmanipulated mice were incubated with either JNK rabbit polyclonal antibody (Santa Cruz Biotechnology) (a–c, e–g, and i–j) or JNK antibody preadsorbed with recombinant JNK protein (d and h). Primary antibody binding was detected with species-matched secondary antibodies and the immunoperoxidase reaction using ABC reagents from Vector Laboratories and diaminobenzidine as the chromagen (brown reaction product). Note the widespread immunostaining of neuronal cell populations in the hippocampus and neocortex. Some neurons showed stronger nuclear staining (g). No cellular immunolabeling was detected with preadsorbed JNK antibody (d and h). (i–j) Neuro-2a cells in culture either were left untreated (i) or irradiated for 10 min with UV-C (j). Pseudocolor indicates increasing levels of immunofluorescence (from left to right) as determined by confocal microscopy. UV-C irradiation resulted in increased nuclear immunostaining for JNK. This was paralleled in replicate cultures by an increase in JNK enzymatic activity (Fig. 1c, Upper). (Bar: 250 μm.)
Activation of JNKs in Response to Environmental Stimuli.
The high levels of JNK activity in the CNS and the widespread neuronal expression of this enzyme suggest that the JNK cascade may be involved in physiological CNS responses. To test this hypothesis, we examined whether widespread increases in neuronal activity are associated with further increases in cerebral JNK activity. Two paradigms were used to alter CNS activity noninvasively: physical restraint (5–10 min) and exploration of a novel environment (10 min). Both of these paradigms elicit widespread increases in neuronal activity but do not result in neuronal injury or death.
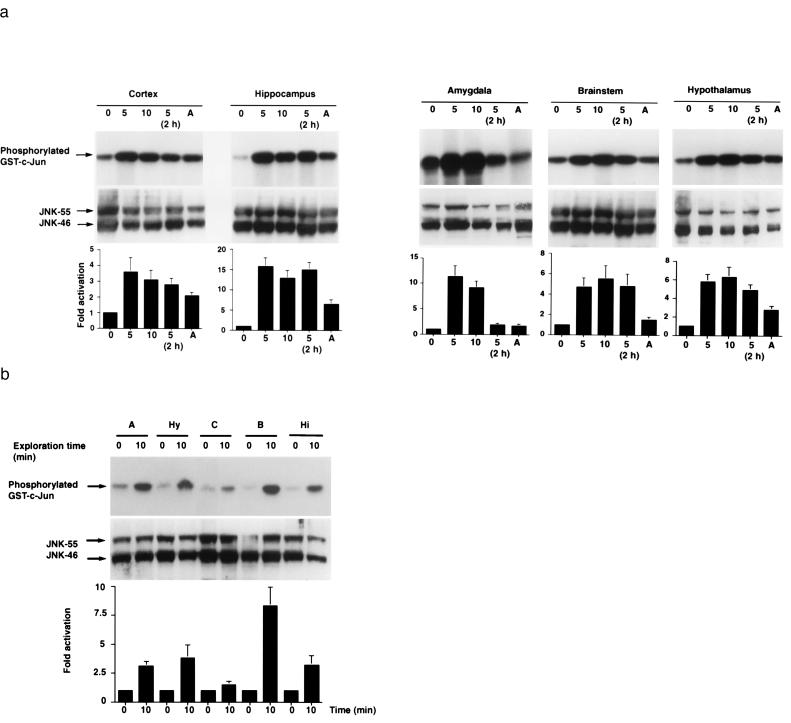
First, we compared mice (n = 3 per group) that were unmanipulated or restrained for 5 or 10 min. Immediately after the restraint or after a 2-hr interval mice were killed by cervical dislocation, and the brains were processed rapidly. Additional unmanipulated mice (n = 3) were euthanized by exposure to metofane vapor. JNK activities and protein levels in different brain regions were quantitated by solid-phase kinase assay and Western blot analysis, respectively. After 5 and 10 min of restraint, JNK activity was increased 3- to 15-fold over baseline levels in all five brain regions analyzed (Fig. 3a). Two hours after 5 min of restraint, JNK activity still remained at an elevated level in the neocortex, hippocampus, brainstem, and hypothalamus but had declined in the amygdala. Anesthesia of unrestrained mice with metofane also activated JNK in the neocortex, hippocampus, and hypothalamus. In contrast, restraint or anesthesia had little effect on cerebral JNK protein levels (Fig. 3a). An in-gel phosphorylation assay revealed that the increased JNK activities in the CNS resulted specifically from an activation of JNK-55 and JNK-46 (data not shown).
Figure 3.
Noninvasive environmental stimulation induces prominent JNK activation in mouse brain. (a) Physical restraint. Mice were unmanipulated (0) or restrained for 5 min (5), 10 min (10), or for 5 min followed by a 2-hr recovery period [5 (2h)]. Additional mice (n = 3) were killed by metofane anesthesia (A). (Top) Representative autoradiographs revealing JNK activities in five of the brain regions analyzed. (Middle) Western blot analysis of JNK protein levels in same tissue lysates. (Bottom) For each brain region, the average baseline level of JNK activity found in unmanipulated mice (n = 3) was arbitrarily defined as 1.0. Fold activation indicates average increases in JNK activity over baseline levels determined by PhosphorImager quantitations of signals obtained in manipulated mice (n = 3/group) in the same kinase assay (error bars = SD). Similar results were obtained in two additional independent kinase assays. (b) Exploration of a novel environment. Control mice were set down into the new cage for only 2–3 sec and returned immediately to their home cage for a 10-min observation period (0), whereas experimental mice were allowed to explore the new environment for 10 min (10). Representative autoradiographs revealing JNK activities (Top) and JNK protein levels (Middle), respectively. (Bottom) For each brain region, the mean level of JNK activity found in control mice (n = 4) was arbitrarily defined as 1.0. Fold activation indicates average increases in JNK activity over control levels determined by PhosphorImager quantitations of signals obtained in experimental mice (n = 4/group) (error bars = SD). Similar results were obtained in an additional independent kinase assay.
Next, we determined whether exploration of a new environment also would increase neuronal JNK activity. Mice were placed into a new cage containing stimulating items they had never encountered before (see Materials and Methods). Control mice (n = 4) were returned immediately to their home cage for 10 min, whereas experimental mice (n = 4) were allowed to explore the new cage for 10 min. After returning to their home cage, control mice quickly settled down into a corner of the cage and rested there for the remainder of the observation period. Experimental mice immediately began to explore the new cage and continued to do so throughout the exploration period. At the end of the observation period, all mice were removed gently from their cages and killed rapidly by cervical dislocation. Brains were removed immediately and analyzed. Whereas control mice had JNK activity levels similar to those of unmanipulated mice, experimental mice showed a strong increase in JNK activity levels in the hippocampus, amygdala, hypothalamus, neocortex, and brainstem (Fig. 3b). In the exploration paradigm the increase in JNK activity was highest in the brainstem (8-fold), whereas after physical restraint it was highest in the hippocampus and amygdala (16- and 11-fold, respectively).
Lack of ERK Activation After Physical Restraint.
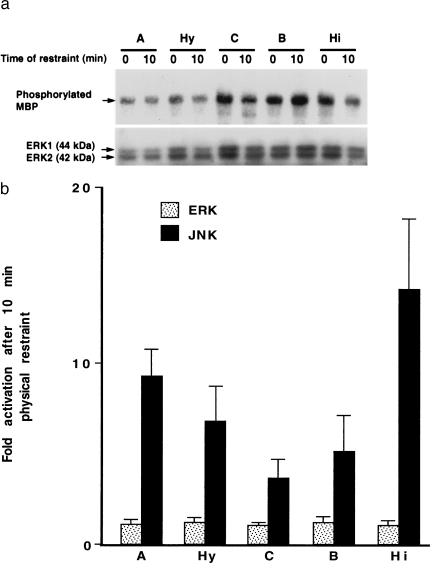
JNKs and ERKs belong to the same mitogen-activated protein kinase family and share upstream kinases. Furthermore, some upstream signals that can activate the JNK cascade also can activate the ERK cascade (24, 25). We therefore looked for evidence of ERK activation in the CNS of unmanipulated and experimentally manipulated mice by immunocomplex kinase assay. After 10 min of restraint, no significant ERK activation over baseline levels could be detected in the neocortex, amygdala, hippocampus, brainstem, or hypothalamus, whereas JNK activities were greatly increased (Fig. 4). These results suggest that the JNK and ERK cascades are regulated by different upstream pathways in CNS neurons, at least in response to the experimental manipulations used here.
Figure 4.
Lack of ERK activation in restrained mice. Mice were unmanipulated (0) or restrained for 10 min (10). ERK and JNK activities in their brain tissue lysates were analyzed by immune complex kinase assay and solid phase kinase assay, respectively. (a) Representative autoradiograph revealing ERK enzymatic activity (Upper) [phosphorylated myelin basic protein (MBP)] and ERK protein levels (Lower) (ERK1 and ERK2) in the amygdala (A), hypothalamus (Hy), neocortex (C), brainstem (B), and hippocampus (Hi). (b) Comparison of ERK activities and JNK activities in different brain regions in response to physical restraint. For each brain region and enzyme, the average baseline level of kinase activity found in unmanipulated mice (n = 3) was arbitrarily defined as 1.0. Fold activation indicates the average increase in kinase activities over baseline levels determined by PhosphorImager quantitations of signals obtained in restrained mice (n = 3) (error bars = SD). Similar results were obtained in two additional independent kinase assays.
DISCUSSION
Our study demonstrates that JNK activity is substantially higher in the CNS than in peripheral organs and that the JNK cascade can be rapidly and strongly activated throughout the brain by noninvasive stimulation such as physical restraint and exploration of a new environment. These observations, together with the expression of JNKs in diverse neuronal subpopulations throughout the brain (Fig. 2) (22), raise the possibility that the JNK cascade functions as an important physiological signal transduction pathway in the CNS.
Upon activation, JNKs translocate into the nucleus (Fig. 2 i and j) (23), where they may phosphorylate and activate nuclear transcription factors. Physiological substrates for JNKs include c-Jun (5), ATF2 (26), and Elk-1 (23). JNK-mediated phosphorylation of these factors promotes their transactivation, which in turn up-modulates the expression of a variety of genes. C-fos, for example, has been extensively characterized as an early gene marker that responds to various types of CNS stimulation (for review see refs. 27 and 28). Elk-1 phosphorylated by JNK can strongly activate the c-fos promoter (23, 29). The transcription factors c-Jun and ATF2 also appear to play an important role in neuronal gene regulation and CNS functions (28, 30). Taken together, the above studies indicate that, as key upstream kinases, JNKs could play a critical regulating role in a variety of physiological CNS functions mediated by these transcription factors.
Ten JNK isoforms have been identified in human brain corresponding to alternatively spliced isoforms derived from JNK1, JNK2, and JNK3 genes (7). As demonstrated here, noninvasive environmental stimuli can dramatically increase overall JNK activity in different regions of the brain. Because no isoform-specific antibodies or inhibitors are available, we could not determine whether this increase in JNK activity reflects the selective overexpression or activation of specific JNK isoforms.
As indicated above, physiological effects of JNK activation could be mediated by phosphorylation and activation of downstream transcription factors in the nucleus. It therefore is possible that the specific JNK immunoreactivity observed in the neuronal cytoplasm and processes (Fig. 2 c and g) reflects primarily nonactivated forms of JNK. However, it is interesting to note in this context that ERKs may participate in cell cycle regulation via phosphorylation of cytoskeletal proteins (31). This raises the intriguing question of whether cytoplasmic proteins also may serve as substrates for JNKs. Recently, it has been shown that purified recombinant JNK can phosphorylate neuronal microtubule-associated protein tau in a cell-free system, suggesting that JNKs may contribute to the formation of neurofibrillary tangles in Alzheimer disease. Unlike other tau kinases such as glycogen synthase kinase-3, JNKs readily phosphorylate Thr205 and Ser422 of tau, which are more highly phosphorylated in Alzheimer tau than in fetal or normal adult tau (32). In view of these findings, it is possible that overstimulation of the JNK cascade could contribute to neurodegenerative diseases. However, in contrast to what might be expected from results obtained in neuronal culture (14, 15), our findings indicate that even prominent activation of the JNK cascade in CNS neurons does not inevitably result in neuronal apoptosis in vivo: in response to environmental stimuli that do not induce neurodegeneration, the overall activity of JNKs in brain regions increased up to 15-fold within 5 min. In cortex, hippocampus, brainstem, and hypothalamus the activity remained at a high level for at least 2 hr after physical restraint. These in vivo findings are in stark contrast to the apoptosis-inducing effect of acute neuronal JNK activation in vitro (14, 15). Dissociation between the JNK cascade and apoptotic pathways recently also has been noticed in fibroblast cultures treated with tumor necrosis factor α (33, 34).
One might speculate that detrimental JNK effects might be prevented in the CNS by a parallel activation of the ERK cascade, which has been proposed to oppose the apoptosis-inducing JNK cascade in vitro (15). This possibility may be all the more appealing given that ERKs are expressed widely in adult CNS neurons (35), and ERKs can be activated in the hippocampus by electrically induced seizures (36, 37). However, our data (Fig. 4) indicate that parallel activation of the ERK cascade does not account for the failure of prominent JNK activation to induce widespread neuronal apoptosis in vivo.
In conclusion, the above results demonstrate that noninvasive environmental stimuli that do not result in neuronal apoptosis can induce a prominent activation of the JNK cascade in the CNS. These findings suggest that JNK may play an important physiological role in neuronal activities. Future studies will need to dissect the cascade of events that lead from environmental change to JNK activation in the CNS and determine the precise role neuronal JNK activation fulfills in CNS functions.
Acknowledgments
We thank M. Buttini and R.V. Farese, Jr. for advice on immunostaining and preparation of photomicrographs; C. Westland and I. Samuels for technical support; S. Ordway for editorial assistance; R. Haines for help with the preparation of the manuscript; and R. Mahley, R. Messing, and B. Conklin for critical reading of the paper. This work was supported by National Institutes of Health Grant AG11385 (L.M.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: JNK, c-Jun N-terminal kinase; CNS, central nervous system; ERK, extracellular signal-regulated kinase.
References
- 1.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 2.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 3.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Pagès G, Pouyssègur J. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 5.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Dérijard B, Davis R J. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Gorospe M, Yang C, Holbrook N J. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 9.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 10.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 11.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 12.Sluss H K, Barrett T, Dérijard B, Davis R J. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovits-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 14.Park D S, Stefanis L, Yan C Y I, Farinelli S E, Greene L A. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 15.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 16.Heasley L E, Storey B, Fanger G R, Butterfield L, Zamarripa J, Blumberg D, Maue R A. Mol Cell Biol. 1996;16:648–656. doi: 10.1128/mcb.16.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westwick J K, Leffert H L, Brenner D A. J Clin Investi. 1995;95:803–810. doi: 10.1172/JCI117730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Heidenreich O, Kitajima I, McGuire K, Li Q, Su B, Nerenberg M. Oncogene. 1996;13:135–142. [PubMed] [Google Scholar]
- 19.Raitano A B, Leffert H J, Hambuch T M, Sawyers C L. Proc Natl Acad Sci USA. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raber J, Toggas S M, Lee S, Bloom F E, Epstein C J, Mucke L. Virology. 1996;226:362–373. doi: 10.1006/viro.1996.0664. [DOI] [PubMed] [Google Scholar]
- 21.Rockenstein E M, McConlogue L, Tan H, Gordon M, Power M, Masliah E, Mucke L. J Biol Chem. 1995;270:28257–28267. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]
- 22.Carletti R, Tacconi S, Bettini E, Ferraguti F. Neuroscience. 1995;69:1103–1110. doi: 10.1016/0306-4522(95)00284-p. [DOI] [PubMed] [Google Scholar]
- 23.Cavigelli M, Dolfi F, Claret F-X, Karin M. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blank J L, Gerwins P, Elliott E M, Sather S, Johnson G L. J Biol Chem. 1996;271:5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 25.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Campbell D, Derijard B, Davis R J. Science. 1995;267:289–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 27.Pacak K, Palkovist M, Kopin I J, Goldstein D S. Front Neuroendocrinol. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- 28.Pennypacker K R, Hong J S, McMillian M K. Trends Pharmacol Sci. 1995;16:317–321. doi: 10.1016/s0165-6147(00)89061-6. [DOI] [PubMed] [Google Scholar]
- 29.Whitmarsh A J, Shore P, Sharrrocks A D, Davis R J. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 30.Reimold A M, Grusby M J, Kosaras B, Fries J W, Mori R, Maniwa S, Clauss I M, Collins T, Sidman R L, Glimcher M J. Nature (London) 1996;379:262–265. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- 31.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 32.Reynolds C H, Utton M A, Gibb G M, Yates A, Anderton B H. J Neurochem. 1997;64:1736–1744. doi: 10.1046/j.1471-4159.1997.68041736.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z-G, Hsu H L, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z-G, Baskaran R, Lea-Chou E T, Wood L D, Chen Y, Karin M, Wang J Y J. Nature (London) 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 35.Jacobowitz D M, Winsky L, Detera-Wadleigh S D. Histochem Cell Biol. 1996;106:303–310. doi: 10.1007/BF02473240. [DOI] [PubMed] [Google Scholar]
- 36.Baraban J M, Fiore R S, Sanghera J S, Paddon H B, Pelech S L. J Neurochem. 1993;60:330–336. doi: 10.1111/j.1471-4159.1993.tb05855.x. [DOI] [PubMed] [Google Scholar]
- 37.Kang U G, Hong K S, Jung H Y, Kim Y S, Seong Y-S, Yang Y C, Park J-B. J Neurochem. 1994;63:1979–1982. doi: 10.1046/j.1471-4159.1994.63051979.x. [DOI] [PubMed] [Google Scholar]