Abstract
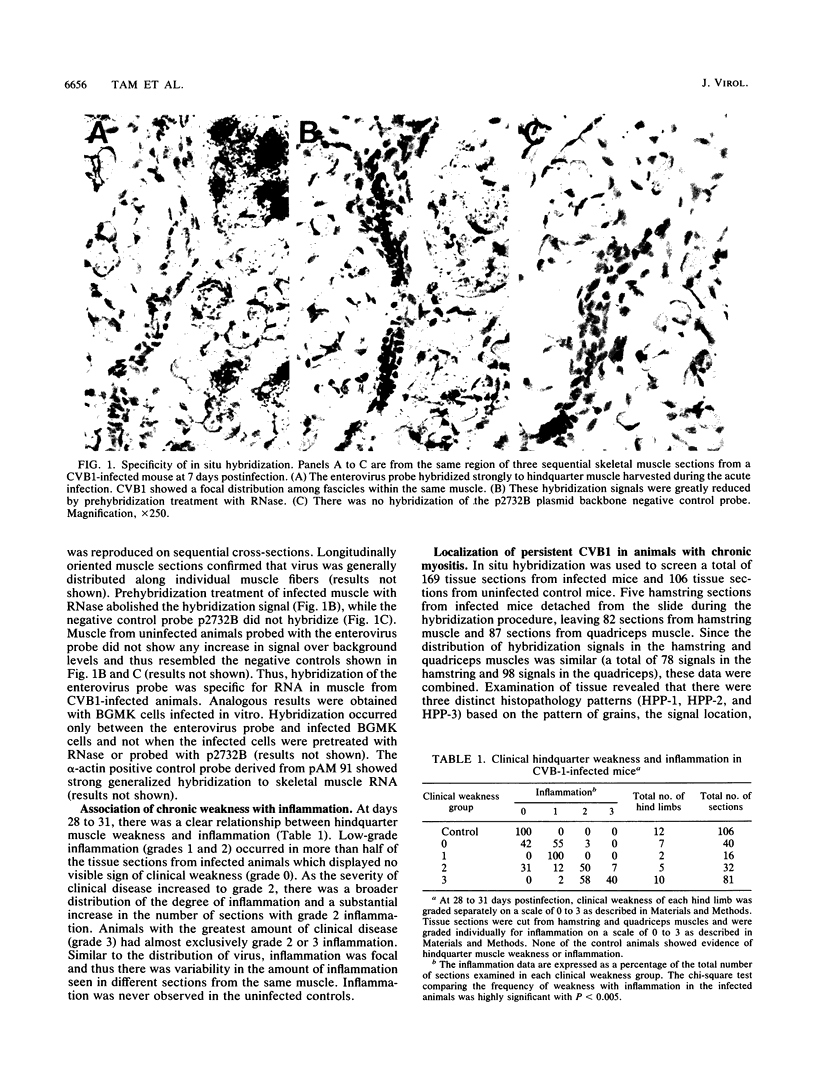
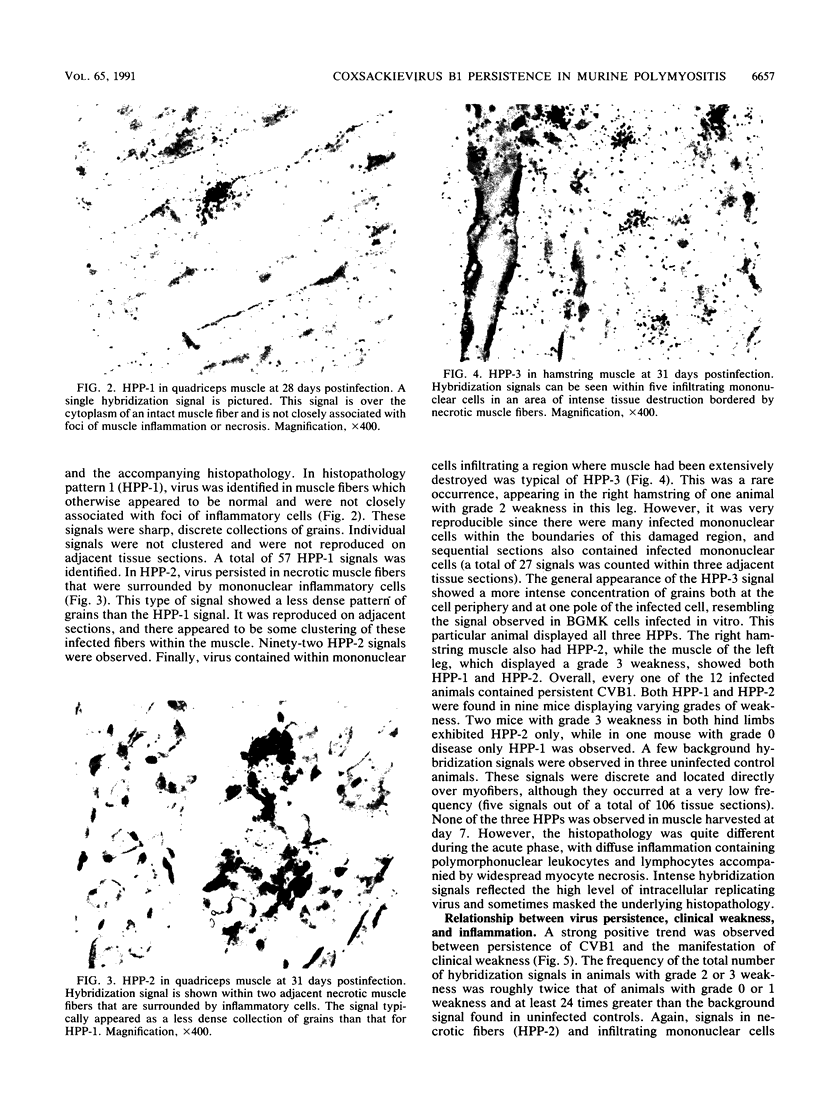
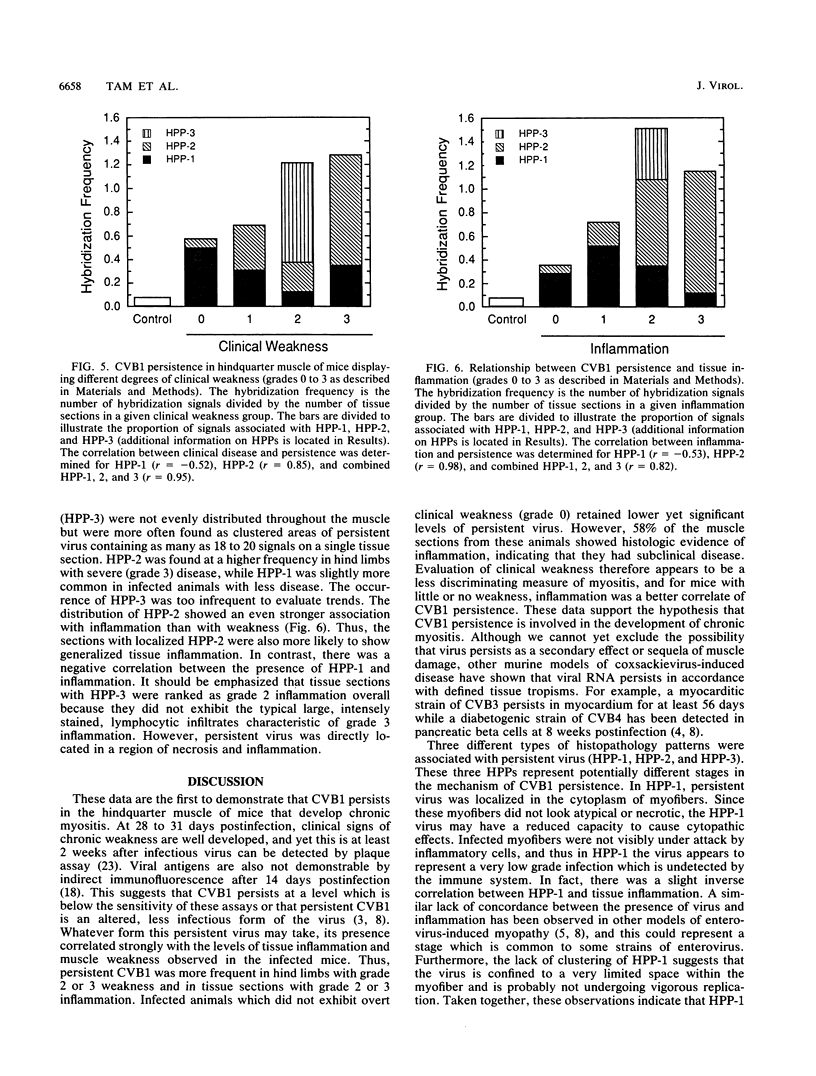
Mice infected with coxsackievirus B1 (CVB1) develop a chronic hindquarter muscle weakness which resembles human polymyositis. In this study, we used in situ hybridization to screen for persistent viral RNA in hamstring and quadriceps muscles from mice that displayed various degrees of clinical weakness. At 28 to 31 days postinfection, when chronic myositis is well developed but infectious virus can no longer be recovered, persistent CVB1 RNA was found in hindquarter skeletal muscle of all 12 infected animals examined. Persistent CVB1 showed a multifocal distribution within muscle and was associated with three different histopathology patterns (HPPs). These three HPPs (HPP-1, HPP-2, and HPP-3) represent potentially different stages in the mechanism of persistence. They are based on the pattern of grains, the location of hybridization signal within the muscle, and the accompanying histopathology. In HPP-1, virus persisted in nonnecrotic muscle fibers and was not directly associated with foci of inflammatory cells. HPP-2 consisted of virus contained within necrotic myocytes that were surrounded by inflammatory cells. HPP-3 was rare and showed virus inside infiltrating mononuclear cells in a region where muscle tissue had been extensively destroyed. Persistent CVB1 occurred more frequently in severely diseased animals and in tissue sections displaying intense inflammation. Moreover, HPP-2 showed a stronger association with tissue inflammation and hindquarter weakness than did HPP-1. These data demonstrate that CVB1 persists in skeletal muscle for at least 28 to 31 days postinfection and support the concept that this persistence plays a role in the development of murine polymyositis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocharov E. V., Shalaurova O. V. Persistence of Coxsackie B1 virus in Balb/C mice. Acta Virol. 1984 Jul;28(4):345–345. [PubMed] [Google Scholar]
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Nejman C. Insulin mRNA content in pancreatic beta cells of coxsackievirus B4-induced diabetic mice. Mol Cell Endocrinol. 1988 Feb;55(2-3):193–202. doi: 10.1016/0303-7207(88)90134-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Nejman C. Membrane-bound virions of coxsackievirus B4: cellular localization, analysis of the genomic RNA, genome-linked protein, and effect on host macromolecular synthesis. Arch Virol. 1985;84(1-2):105–118. doi: 10.1007/BF01310557. [DOI] [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyypiä T., Stålhandske P., Vainionpä R., Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984 Mar;19(3):436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. G., Minnich L. L., Johnson P. C. Selective polymyositis inducted by coxsackievirus B1 in mice. J Infect Dis. 1979 Aug;140(2):239–243. doi: 10.1093/infdis/140.2.239. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. L., Rotbart H. A., Abzug M. J., Ringel S. P., Levin M. J. Evidence for a novel picornavirus in human dermatomyositis. Ann Neurol. 1989 Aug;26(2):204–209. doi: 10.1002/ana.410260204. [DOI] [PubMed] [Google Scholar]
- Schiraldi O., Iandolo E. Polymyositis accompanying coxsackie virus B2 infection. Infection. 1978;6(1):32–34. doi: 10.1007/BF01641089. [DOI] [PubMed] [Google Scholar]
- Schnurr D. P., Schmidt N. J. Coxsackievirus B3 persistence and myocarditis in NFR nu/nu and +/nu mice. Med Microbiol Immunol. 1984;173(1):1–7. doi: 10.1007/BF02123563. [DOI] [PubMed] [Google Scholar]
- Strongwater S. L., Dorovini-Zis K., Ball R. D., Schnitzer T. J. A murine model of polymyositis induced by coxsackievirus B1 (Tucson strain). Arthritis Rheum. 1984 Apr;27(4):433–442. doi: 10.1002/art.1780270411. [DOI] [PubMed] [Google Scholar]
- Tourtellotte W. W., Verity A. N., Schmid P., Martinez S., Shapshak P. Covalent binding of formalin fixed paraffin embedded brain tissue sections to glass slides suitable for in situ hybridization. J Virol Methods. 1987 Feb;15(2):87–99. doi: 10.1016/0166-0934(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Tracy S. Comparison of genomic homologies in the coxsackievirus B group by use of cDNA:RNA dot-blot hybridization. J Clin Microbiol. 1985 Mar;21(3):371–374. doi: 10.1128/jcm.21.3.371-374.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers R. L., Hughes G. R., Cambridge G., Sewell J. R. Coxsackie B neutralisation titres in polymyositis/dermatomyositis. Lancet. 1977 Jun 11;1(8024):1268–1268. doi: 10.1016/s0140-6736(77)92487-4. [DOI] [PubMed] [Google Scholar]
- Yousef G. E., Isenberg D. A., Mowbray J. F. Detection of enterovirus specific RNA sequences in muscle biopsy specimens from patients with adult onset myositis. Ann Rheum Dis. 1990 May;49(5):310–315. doi: 10.1136/ard.49.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytterberg S. R. Coxsackievirus B 1 induced murine polymyositis: acute infection with active virus is required for myositis. J Rheumatol. 1987 Feb;14(1):12–18. [PubMed] [Google Scholar]
- Ytterberg S. R., Mahowald M. L., Messner R. P. Coxsackievirus B 1-induced polymyositis. Lack of disease expression in nu/nu mice. J Clin Invest. 1987 Aug;80(2):499–506. doi: 10.1172/JCI113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytterberg S. R., Mahowald M. L., Messner R. P. T cells are required for coxsackievirus B1 induced murine polymyositis. J Rheumatol. 1988 Mar;15(3):475–478. [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Bowles N. E., Archard L. C., Mann G. F., Mowbray J. F. Detection of enterovirus RNA in experimentally infected mice by molecular hybridisation: specificity of subgenomic probes in quantitative slot blot and in situ hybridisation. J Med Virol. 1988 Dec;26(4):375–386. doi: 10.1002/jmv.1890260405. [DOI] [PubMed] [Google Scholar]