Abstract
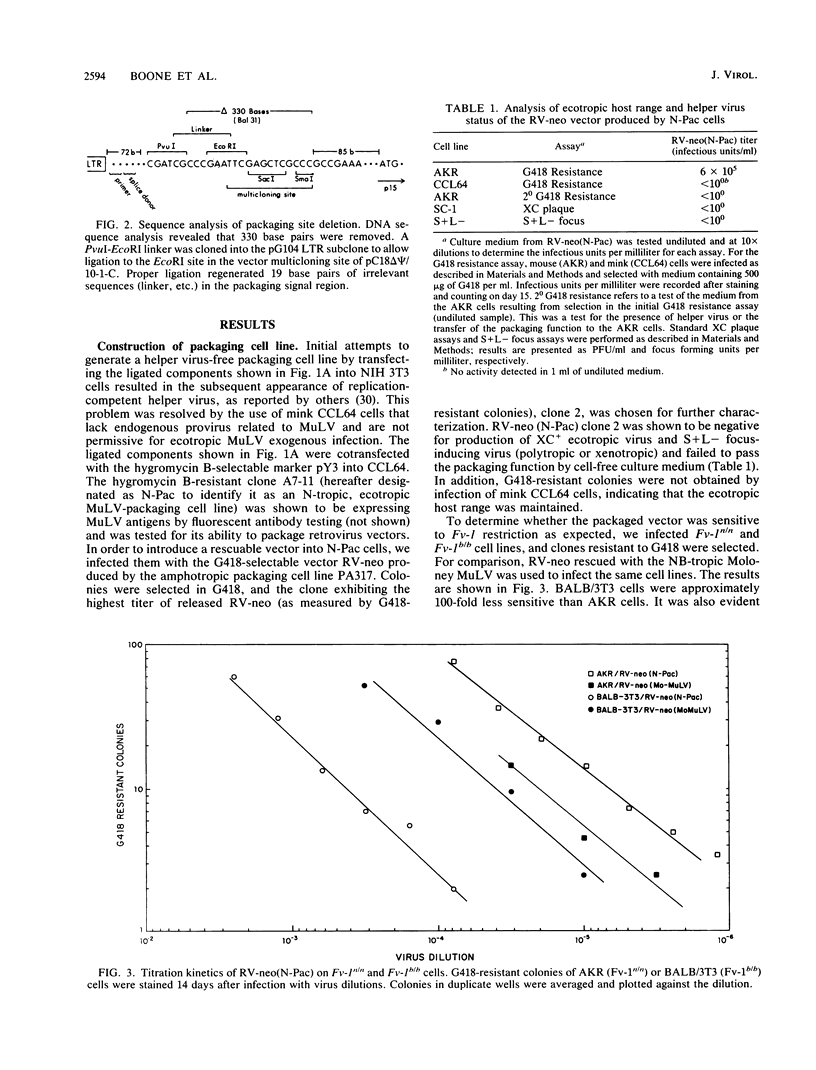
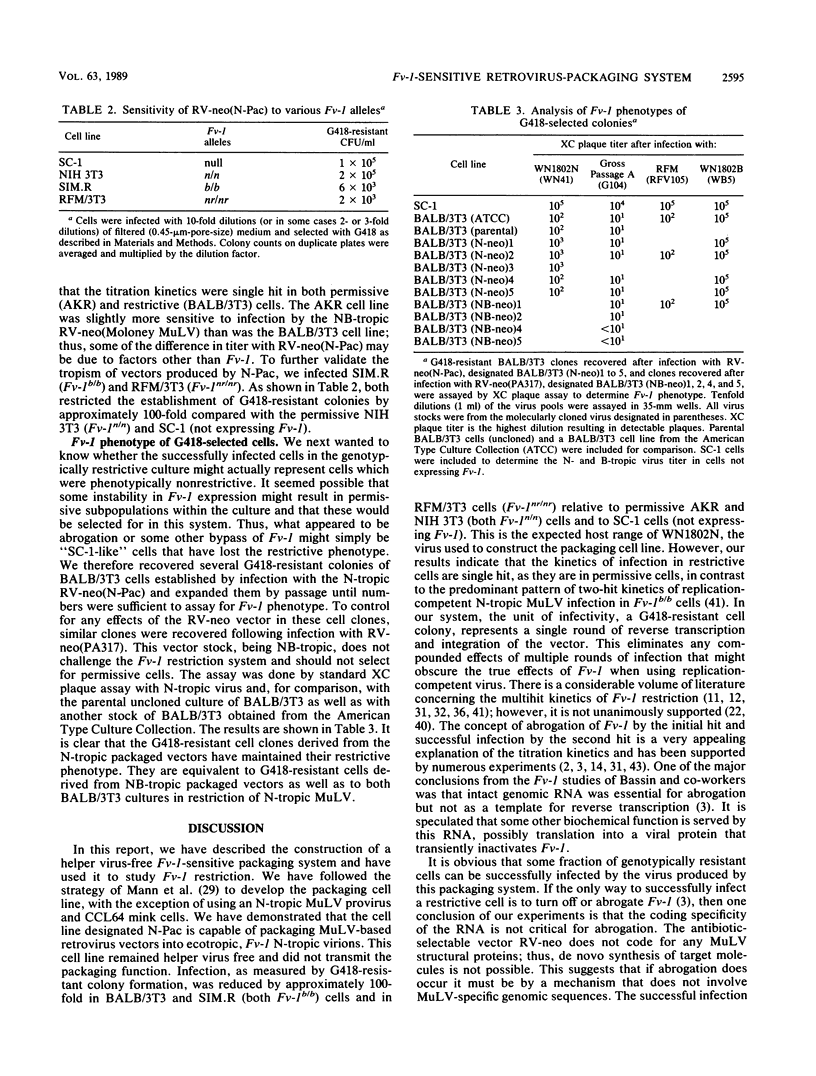
We have constructed an RNA-packaging-deficient mutant of N-tropic murine leukemia virus WN1802N by removal of 330 nucleotides located between the upstream long terminal repeat and the start of the gag gene region. Transfection into mink CCL64 cells produced a cell line capable of packaging retrovirus vectors into ecotropic, Fv-1 N-tropic virions. Using retrovirus vectors that confer resistance to the antibiotic G418, we demonstrated that the magnitude of restriction in BALB/3T3 and SIM.R cells (both Fv-1b/b) and in RFM/3T3 cells (Fv-1nr/nr) is approximately 100-fold compared with that in AKR or NIH 3T3 cells (both Fv-1n/n). Furthermore, titration kinetics were single hit in restrictive cells. Colonies of antibiotic-resistant cells recovered after infection of genotypically restrictive cultures were phenotypically restrictive when reinfected, ruling out selection of stably nonrestrictive subpopulations. These results suggest that the ability to infect some fraction of cells in a genotypically restrictive culture does not require specific abrogation and that multihit kinetics may not be an essential feature of Fv-1 restriction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Duran-Troise G., Gerwin B. I., Rein A. Abrogation of Fv-1b restriction with murine leukemia viruses inactivated by heat or by gamma irradiation. J Virol. 1978 May;26(2):306–315. doi: 10.1128/jvi.26.2.306-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Gerwin B. I., Duran-Troise G., Gisselbrecht S., Rein A. Murine sarcoma virus pseudotypes acquire a determinant specifying N or B tropism from leukaemia virus during rescue. Nature. 1975 Jul 17;256(5514):223–225. doi: 10.1038/256223a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Gerwin B. I., Levin J. G., Duran-Troise G., Benjers B. M., Rein A. Macromolecular requirements for abrogation of Fv-1 restriction by murine leukemia viruses. J Virol. 1980 Aug;35(2):287–297. doi: 10.1128/jvi.35.2.287-297.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Glover P. L., Innes C. L., Niver L. A., Bondurant M. C., Yang W. K. Fv-1 N- and B-tropism-specific sequences in murine leukemia virus and related endogenous proviral genomes. J Virol. 1988 Aug;62(8):2644–2650. doi: 10.1128/jvi.62.8.2644-2650.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Myer F. E., Yang D. M., Ou C. Y., Koh C. K., Roberson L. E., Tennant R. W., Yang W. K. Reversal of Fv-1 host range by in vitro restriction endonuclease fragment exchange between molecular clones of N-tropic and B-tropic murine leukemia virus genomes. J Virol. 1983 Oct;48(1):110–119. doi: 10.1128/jvi.48.1.110-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Fv-1 host restriction of Friend leukemia virus: analysis of unintegrated proviral DNA. J Virol. 1981 Oct;40(1):45–55. doi: 10.1128/jvi.40.1.45-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R., Kopchick J. Fv-1 host cell restriction of friend leukemia virus: microinjection of unintegrated viral DNA. J Virol. 1984 Apr;50(1):271–274. doi: 10.1128/jvi.50.1.271-274.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology. 1975 Jun;65(2):320–332. doi: 10.1016/0042-6822(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Kojola J., Kaplan H. S. New gene locus modifying susceptibility to certain B-tropic murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Feb;73(2):585–590. doi: 10.1073/pnas.73.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Troise G., Bassin R. H., Rein A., Gerwin B. I. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell. 1977 Mar;10(3):479–488. doi: 10.1016/0092-8674(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Mizuuchi K. Retroviral DNA integration: structure of an integration intermediate. Cell. 1988 Aug 12;54(4):497–504. doi: 10.1016/0092-8674(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht S., Bassin R. H., Gerwin B. I., Rein A. Dual susceptibility of A 3T3 mouse cell line to infection by N- and B-tropic murine leukemia virus: apparent lack of expression of the FV-1 gene. Int J Cancer. 1974 Jul 15;14(1):106–113. doi: 10.1002/ijc.2910140113. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto A., Masuda M., Yoshikura H. Synthesis of proviral DNA in inbred mouse-derived clones of cells expressing different Fv-1 phenotypes. J Gen Virol. 1985 Oct;66(Pt 10):2265–2269. doi: 10.1099/0022-1317-66-10-2265. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J Virol. 1975 Dec;16(6):1593–1598. doi: 10.1128/jvi.16.6.1593-1598.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Linney E., Neill S. D., Prestridge D. S. Retroviral vector gene expression in F9 embryonal carcinoma cells. J Virol. 1987 Oct;61(10):3248–3253. doi: 10.1128/jvi.61.10.3248-3253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Decléve A., Kaplan H. S. Conversion of restrictive mouse cells to permissiveness during sequential and mixed double infection by murine leukemia viruses. Virology. 1976 Oct 1;74(1):140–153. doi: 10.1016/0042-6822(76)90136-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Deitch C. J., Pincus T. Multiplicity-dependent kinetics and murine leukemia virus infection in Fv-1-sensitive and Fv-1-resistant cells. Virology. 1976 Aug;73(1):23–35. doi: 10.1016/0042-6822(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Boone L. R., Koh C. K., Tennant R. W., Yang W. K. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J Virol. 1983 Dec;48(3):779–784. doi: 10.1128/jvi.48.3.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh V., Blackstein M. E., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: titration patterns in strains SIM and SIM.R congenic at the Fv-1 locus. J Virol. 1976 May;18(2):473–480. doi: 10.1128/jvi.18.2.473-480.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Otten J. A., Brown A., Yang W. K., Kennel S. J. Characterization of Fv-1 host range strains of murine retroviruses by titration and p30 protein characteristics. Virology. 1979 Dec;99(2):349–357. doi: 10.1016/0042-6822(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Naito Y., Moriwaki K. Unstable resistance of G mouse fibroblasts to ecotropic murine leukemia virus infection. J Virol. 1979 Mar;29(3):1078–1086. doi: 10.1128/jvi.29.3.1078-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Tejima S., Kuchino T., Segawa K., Odaka T. Characterization of N-type and dually permissive cells segregated from mouse fibroblasts whose Fv-1 phenotype could be modified by another independently segregating gene(s). J Virol. 1982 Jan;41(1):145–152. doi: 10.1128/jvi.41.1.145-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. Ultraviolet inactivation of murine leukemia virus and its assay in permissive and non-permissive cells. Int J Cancer. 1973 May;11(3):739–746. doi: 10.1002/ijc.2910110325. [DOI] [PubMed] [Google Scholar]



