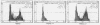Abstract
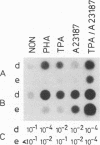
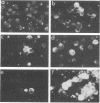
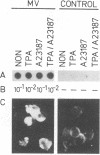
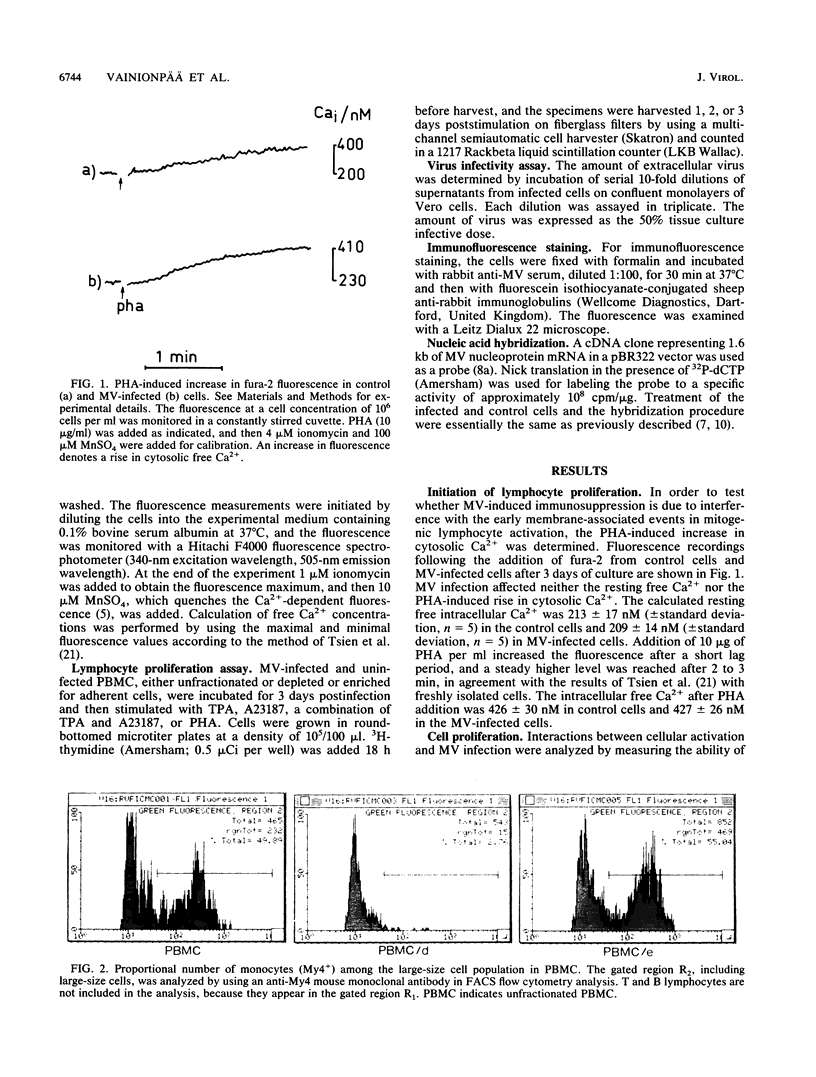
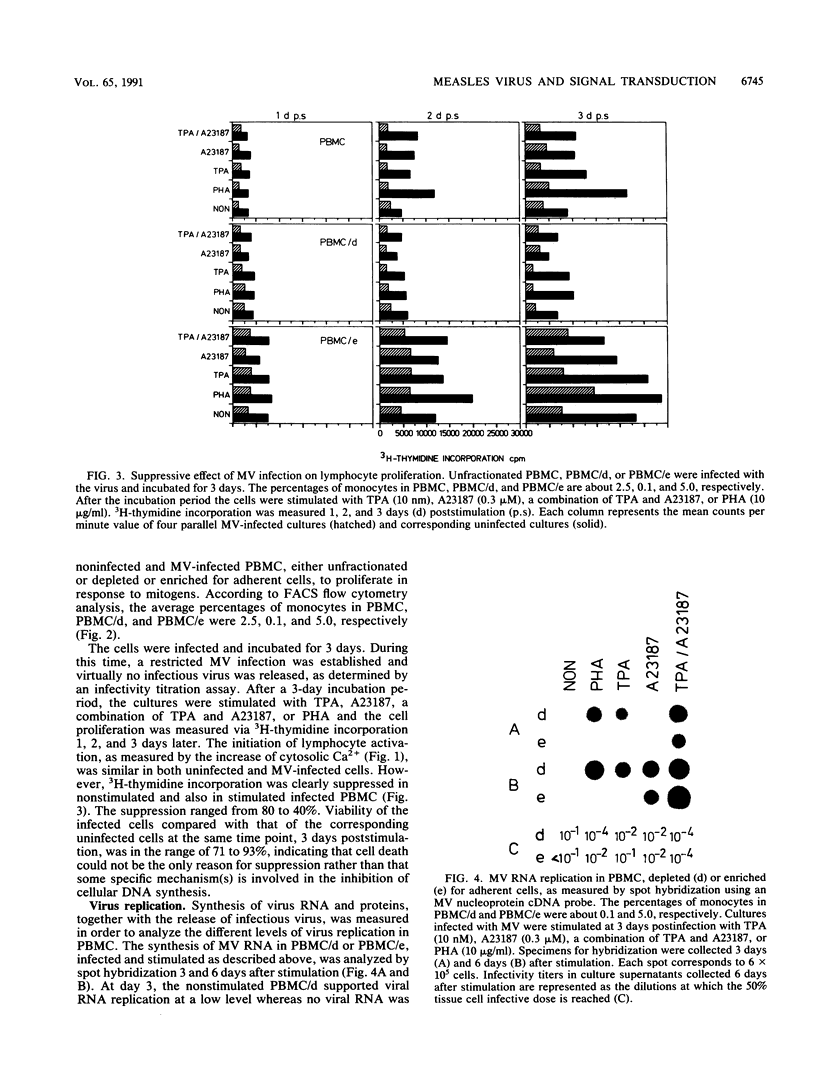
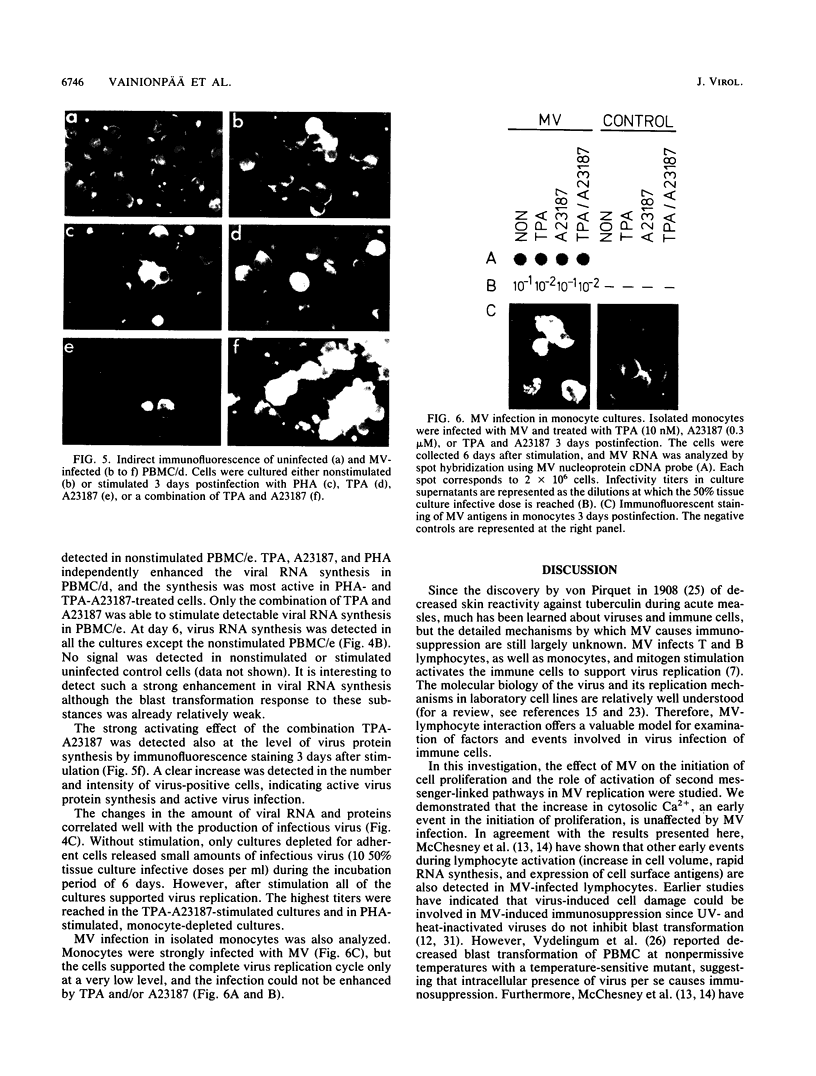
In order to understand measles virus-lymphocyte interactions, we have started to analyze factors and events which regulate measles virus infection in peripheral blood mononuclear cells (PBMC). We analyzed the initiation of cell proliferation, induced by phytohemagglutinin, in infected and control PBMC by measuring intracellular free Ca2+ by using fura-2. Measles virus-infected and control PBMC responded similarly with an increase in the amount of cytosolic free Ca2+, indicating that the early activation events are not affected and are not involved in immunosuppression. The activation signals, Ca2+ and protein kinase C, induced specifically and independently by Ca ionophore A23187 or 12-O-tetradecanoylphorbol-13-acetate (TPA), changed the restricted measles virus infection to a productive one. The combination of TPA and A23187 was the most potent activator of measles virus replication. TPA and A23187 operate through different activation mechanisms, and it is evident that measles virus replication depends on the activation of cellular signal pathways. Depletion of adherent cells enhanced virus replication, especially at the early stage of infection, indicating the inhibitory role of monocytes. Monocytes were strongly infected, but they supported complete measles virus replication only at a very low level, and virus replication could not be enhanced with TPA and/or A23187.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Andersson L. C. Direct mitogenic effect of ionophore A23187 on isolated human T helper lymphocytes. Eur J Immunol. 1984 Mar;14(3):286–288. doi: 10.1002/eji.1830140317. [DOI] [PubMed] [Google Scholar]
- Bohn W., Rutter G., Hohenberg H., Mannweiler K., Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986 Feb;149(1):91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Fournier J. G., Tardieu M., Lebon P., Robain O., Ponsot G., Rozenblatt S., Bouteille M. Detection of measles virus RNA in lymphocytes from peripheral-blood and brain perivascular infiltrates of patients with subacute sclerosing panencephalitis. N Engl J Med. 1985 Oct 10;313(15):910–915. doi: 10.1056/NEJM198510103131502. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Nakashima H., Kobayashi N., Yamamoto N. Tumor promoter, TPA, enhances replication of HTLV-III/LAV. Virology. 1986 Oct 30;154(2):249–258. doi: 10.1016/0042-6822(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Hirsch R. L., Mokhtarian F., Griffin D. E., Brooks B. R., Hess J., Johnson R. T. Measles virus vaccination of measles seropositive individuals suppresses lymphocyte proliferation and chemotactic factor production. Clin Immunol Immunopathol. 1981 Dec;21(3):341–350. doi: 10.1016/0090-1229(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Korkiamäki P., Vainionpä R. Replication of measles virus in human lymphocytes. J Exp Med. 1985 Jun 1;161(6):1261–1271. doi: 10.1084/jem.161.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Protein kinase C and calcium ion in mitogenic response of macrophage-depleted human peripheral lymphocytes. J Biol Chem. 1985 Feb 10;260(3):1366–1369. [PubMed] [Google Scholar]
- Lee J. K., Black J. D., Repasky E. A., Kubo R. T., Bankert R. B. Activation induces a rapid reorganization of spectrin in lymphocytes. Cell. 1988 Dec 2;55(5):807–816. doi: 10.1016/0092-8674(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Linette G. P., Hartzman R. J., Ledbetter J. A., June C. H. HIV-1-infected T cells show a selective signaling defect after perturbation of CD3/antigen receptor. Science. 1988 Jul 29;241(4865):573–576. doi: 10.1126/science.2899908. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Altman A., Oldstone M. B. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J Immunol. 1988 Feb 15;140(4):1269–1273. [PubMed] [Google Scholar]
- McChesney M. B., Kehrl J. H., Valsamakis A., Fauci A. S., Oldstone M. B. Measles virus infection of B lymphocytes permits cellular activation but blocks progression through the cell cycle. J Virol. 1987 Nov;61(11):3441–3447. doi: 10.1128/jvi.61.11.3441-3447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Salonen R., Ilonen J., Salmi A. Measles virus infection of unstimulated blood mononuclear cells in vitro: antigen expression and virus production preferentially in monocytes. Clin Exp Immunol. 1988 Feb;71(2):224–228. [PMC free article] [PubMed] [Google Scholar]
- Stallcup K. C., Raine C. S., Fields B. N. Cytochalasin B inhibits the maturation of measles virus. Virology. 1983 Jan 15;124(1):59–74. doi: 10.1016/0042-6822(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Ehrnst A. Transmembrane communication in cells chronically infected with measles virus. J Cell Biol. 1979 May;81(2):396–402. doi: 10.1083/jcb.81.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainiopä R., Ziola B., Salmi A. Measles virus polypeptides in purified virions and in infected cells. Acta Pathol Microbiol Scand B. 1978 Dec;86B(6):379–385. doi: 10.1111/j.1699-0463.1978.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Vydelingum S., Ilonen J., Salonen R., Marusyk R., Salmi A. Infection of human peripheral blood mononuclear cells with a temperature-sensitive mutant of measles virus. J Virol. 1989 Feb;63(2):689–695. doi: 10.1128/jvi.63.2.689-695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leonard R., Cheynier R., Feldman M., Sarin P. S., Gallo R. C. Long-term cultures of HTLV-III--infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science. 1986 Feb 21;231(4740):850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]
- Zweiman B., Lisak R. P., Waters D., Koprowski H. Effects of purified measles virus components on proliferating human lymphocytes. Cell Immunol. 1979 Oct;47(2):241–247. doi: 10.1016/0008-8749(79)90334-4. [DOI] [PubMed] [Google Scholar]