Abstract
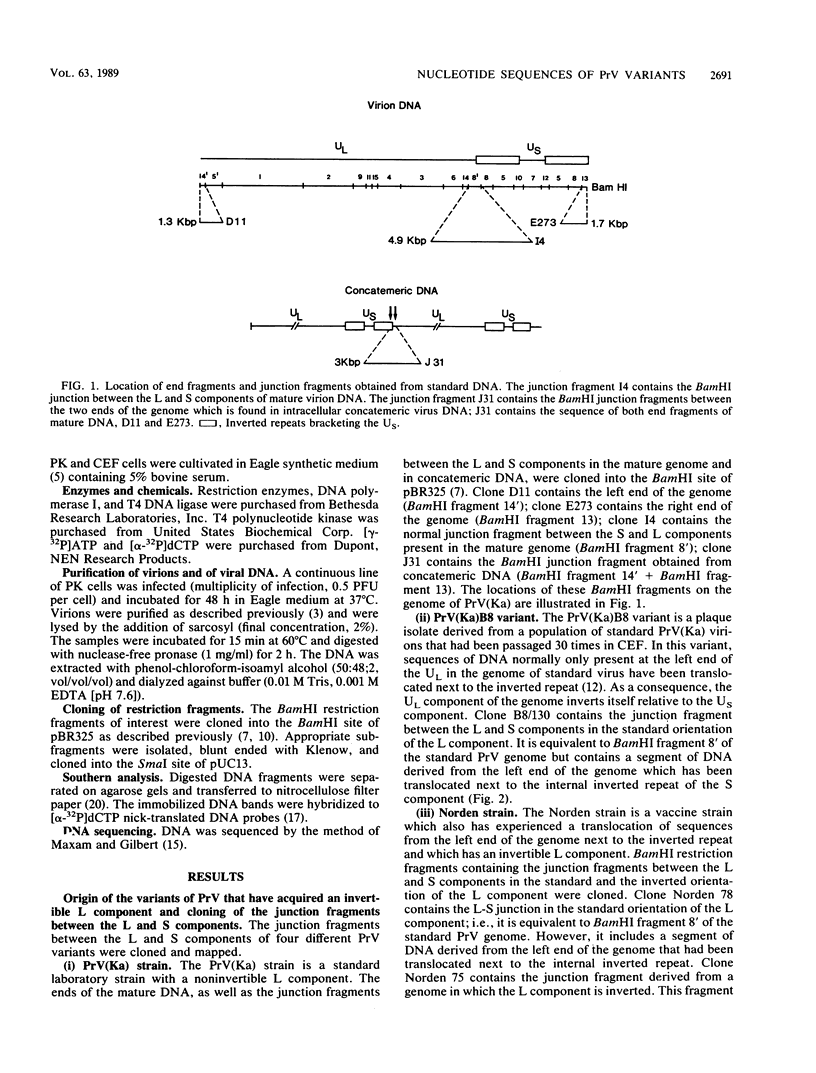
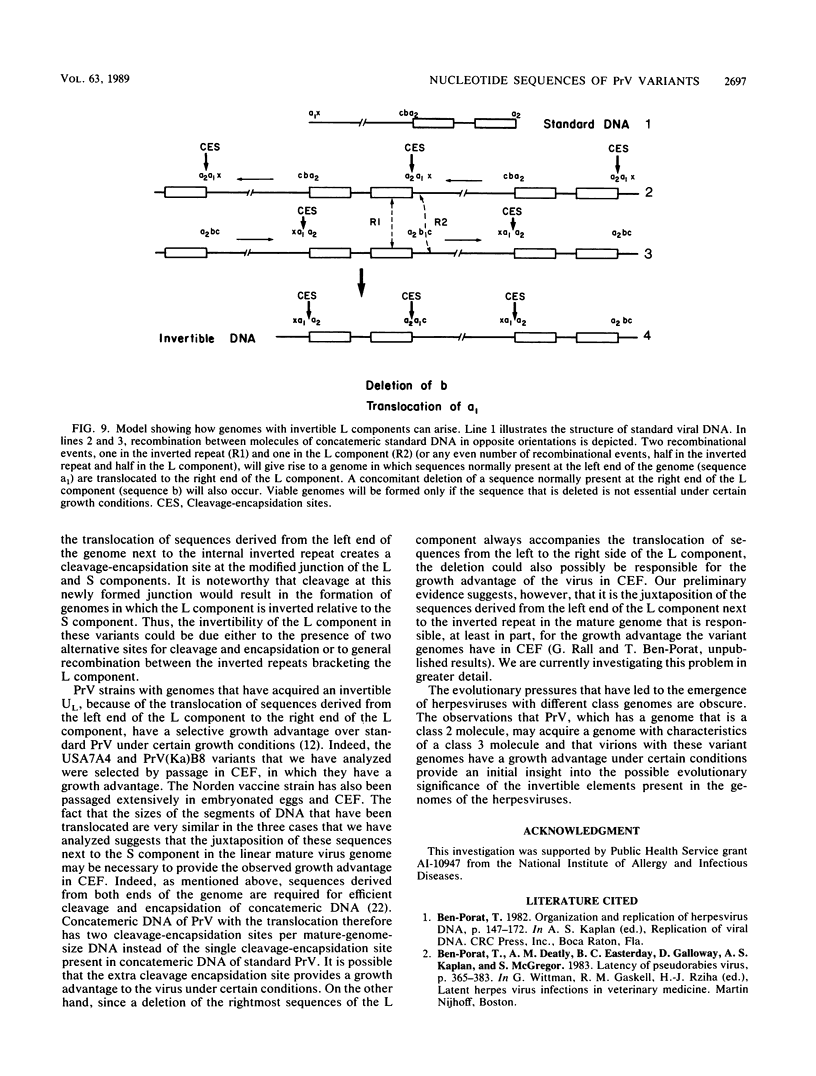
The genome of pseudorabies virus (PrV) consists of two components--a noninvertible long (L) and an invertible short (S) component. The S component is bracketed by inverted repeats. In some variant strains of PrV (which have a selective growth advantage in certain cell lines), a sequence normally present at the left end of the L component has been translocated to the right end of the L component next to the inverted repeat. Consequently, these strains have acquired a genome with an L component that is bracketed by inverted repeats and that also inverts. We have determined the restriction maps and have analyzed the nucleotide sequences of those parts of the genome of three strains with invertible L components that contain the translocated segment of DNA. The results were as follows. The translocated fragments were derived in all cases from the extreme left end of the L component only. The sizes of the translocated fragments were similar, ranging from 1.3 to 1.4 kilobase pairs. The junction between the L and S components in these strains was the same as that in standard viral concatemeric DNA. The translocation of sequences from the left end of the genome next to the inverted repeats was always accompanied by a deletion of sequences from the right end of the L component. The sizes of the deleted fragments varied considerably, ranging from 0.8 to 2.3 kilobase pairs. Sequence homology at the points of recombination, i.e., at the junction between the right end and the left end of the L component, existed sometimes but not always. A model depicting how a virus with a class 2 genome (such as PrV) may acquire a genome with characteristics of a class 3 genome (such as herpes simplex virus) is presented.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Demarchi J. M., Kaplan A. S. Characterization of defective interfering viral particles present in a population of pseudorabies virions. Virology. 1974 Sep;61(1):29–37. doi: 10.1016/0042-6822(74)90239-6. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Rixon F. J., Blankenship M. L. Analysis of the structure of the genome of pseudorabies virus. Virology. 1979 Jun;95(2):285–294. doi: 10.1016/0042-6822(79)90484-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Harper L., Demarchi J., Ben-Porat T. Sequence of the genome ends and of the junction between the ends in concatemeric DNA of pseudorabies virus. J Virol. 1986 Dec;60(3):1183–1185. doi: 10.1128/jvi.60.3.1183-1185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Blankenship M. L., Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984 Mar;49(3):970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Gielkens A., Csobai I., Ben-Porat T. Evolution of pseudorabies virions containing genomes with an invertible long component after repeated passage in chicken embryo fibroblasts. J Virol. 1987 Jun;61(6):1772–1780. doi: 10.1128/jvi.61.6.1772-1780.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Kaplan A. S., Ben-Porat T. Multiple defects in the genome of pseudorabies virus can affect virulence without detectably affecting replication in cell culture. Virology. 1987 Nov;161(1):181–189. doi: 10.1016/0042-6822(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Platt K. B., Maré C. J., Hinz P. N. Differentiation of vaccine strains and field isolates of pseudorabies (Aujeszky's disease) virus: thermal sensitivity and rabbit virulence markers. Arch Virol. 1979;60(1):13–23. doi: 10.1007/BF01318093. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevely W. S. Inverted repetition in the chromosome of pseudorabies virus. J Virol. 1977 Apr;22(1):232–234. doi: 10.1128/jvi.22.1.232-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Harper L., Ben-Porat T. cis Functions involved in replication and cleavage-encapsidation of pseudorabies virus. J Virol. 1986 Aug;59(2):318–327. doi: 10.1128/jvi.59.2.318-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



