Abstract
We report herein the successful long term engraftment of highly purified hematopoietic stem cells (HSCs) without any facilitating cells in fully allogeneic recipient mice across the entire major histocompatibility complex (MHC) transplantation barrier. This finding challenges the assumption that highly purified marrow HSCs alone cannot produce long-lived allogeneic bone marrow chimeras across the MHC barrier. In the present experiments, 1 × 105 HSCs from 5-fluorouracil (5-FU)-treated donors, without any facilitating cells, have been found to repopulate lethally irradiated fully allogeneic recipients. Low density, lineage-negative (CD4−, CD8−, B220−, Mac-1−, Gr-1−), CD71-negative, class I highly positive, FACS-sorted cells from 5-FU-treated C57BL/6 (B6) donor mice were transplanted into lethally irradiated BALB/c recipients. (BALB/c → BALB/c) → BALB/c T cell-depleted marrow cells used as compromised cells were also transplanted into the recipients to permit experiments to be pursued over a long period of time. Cells of donor origin in all recognized lineages of hematopoietic cells developed in these allogeneic chimeras. One thousand HSCs were sufficient to repopulate hemiallogeneic recipients, but 1 × 104 HSCs alone from 5-FU-treated donors failed to repopulate the fully allogeneic recipients. Transplantation of primary marrow stromal cells or bones of the donor strain into recipient, together with 1 × 104 HSCs, also failed to reconstitute fully allogeneic recipients. Suppression of resistance of recipients by thymectomy or injections of granulocyte colony-stimulating factor before stem cell transplantation enhanced the engraftment of allogeneic HSCs. Our experiments show that reconstitution of all lymphohematopoietic lineages across the entire MHC transplantation barriers may be achieved by transplanting allogeneic HSCs alone, without any facilitating cells, as long as a sufficient number of HSCs is transplanted.
Over the last decade, purification and characterization of hematopoietic stem cells (HSCs) have progressed rapidly (1–7). With respect to in vivo definitions, primitive hematopoietic stem cells may be identified as cells that exhibit capacity for long term lymphohematopoietic reconstitution and continuous self-renewal (4, 5). Ogata et al. (1) have carried out investigations that have established for mice the characteristic markers of the primitive hematopoietic stem cells and permit their ready isolation for experimental trials. Their investigations showed that HSCs are cells of low density (LD), lineage-negative, CD71−, and major histocompatibility complex (MHC) class Ibright. They are also wheat germ agglutinin-positive, Thy-1low, Sca-1+, and c-kit-negative or low. These investigations have shown that even a single HSC or very few such cells can reconstitute cells of all major lineages of the hematopoietic and lymphoid systems in male to female syngeneic transplantation. Further, the cells in question are truly long term repopulating units. Osawa et al. (6) reported that a single CD34low/negative HSC can achieve long term reconstitution in congeneic mice. They used a competitive long term reconstitution analysis of mouse strains congeneic for different alleles of the Ly-5 antigen on the C57BL/6 background. CD34+, c-kit+, Sca-1+, lineage− cells revealed early but unsustained multilineage hematopoietic reconstitution. By contrast, delayed but long term multilineage reconstitution was observed after transplantation of CD34low/−, c-kit+, Sca-1+, lineage− cells. Recently, Doi et al. (7) have shown that the true fully self-renewing HSCs are cells of low density that are 5-fluorouracil (5-FU)-resistant, c-kit<low, MHC class Ihigh, CD71−. These cells not only can reconstruct cells in every hematopoietic and lymphoid lineage up to 1 year after transplantation into irradiated syngeneic recipients but are also capable of reconstituting hematopoiesis and lymphopoiesis and of restoring immune competence over at least two serial passages. However, all these experiments have been done with MHC-compatible combinations.
El-Badri and Good (8) showed that wheat germ-positive, LD stem cell preparations from allogeneic donors can achieve impressive lymphohematopoietic reconstitution if the stem cell transplantations (SCTs) are made within but not across the MHC barriers. Kaufman et al. (9) have described a facilitating cell that fosters hematopoietic and immunologic development even across MHC barriers. The facilitating cell they described is CD8+, CD3+, CD45R+, Thy-1+, class IIdim/intermediate but αβ-TCR− and γδ-TCR−. El-Badri et al. (10) also reported that osteoblasts from the donor strain can facilitate engraftment of fully allogeneic HSCs in mice. However, there has thus far been no report concerning reconstitution of fully allogeneic recipients after supralethal irradiation by using highly purified HSC alone without facilitating cells. We show herein that highly purified HSC alone can repopulate every lymphohematopoietic lineage in lethally irradiated allogeneic recipient mice across the entire MHC transplantation barrier and that such stem cell grafts can reconstitute hematopoiesis over a long term.
MATERIALS AND METHODS
Mice.
Female BALB/c, C57BL/6 (B6 Ly-5.2), congeneic C57BL/6-Ly-5.1 (B6 Ly-5.1), and (B6 × C3H) F1 mice, 7–8 weeks old, were purchased from The Jackson Laboratory and maintained in a pathogen-free environment. All studies using animals were conducted in American Association of Laboratory Animal Care-accredited facilities, in compliance with the principles of the Guide for the Care and Use of Laboratory Animals.
Antibodies.
All of the mAbs used in this study were purchased from PharMingen. Purified rat mAbs against CD4, CD8, CD45R (B220), Gr-1, Mac-1, and CD71 were used for depleting lineage-positive and transferrin receptor-positive cells and obtaining purified HSC populations. Fluorescein isothiocyanate (FITC)-conjugated mAbs against H-2Kb, H-2Kd, Ly-5.1, and Ly-5.2 were used for chimerism assay. Phycoerythrin-conjugated mAbs against B220, Thy-1.2, or Mac-1 and biotinylated Gr-1 plus Streptavidin-Cy-Chrome were used for analyzing cell surface phenotype.
Purification of HSCs.
Highly purified HSCs were marrow cells obtained from appropriate donor mice that had first been treated with 5-FU (SoloPak Laboratories, Franklin Park, IL) given i.v. in a dose of 150 mg/kg, 4 days before marrow harvesting. The HSCs were isolated as cells of LD by Percoll (Pharmacia) discontinuous density gradient fractionation (1.059 < P < 1.071). They were lineage-negative as was achieved by eliminating lineage-positive cells and transferrin receptor (CD71)-positive cells by using specific mAbs (anti-CD4, CD8, B220, Gr-1, Mac-1, and CD71) and removing these lineage-positive cells by immunomagnetic beads (Dynabeads, Dynal, Oslo, Norway). The HSCs are then selected as cells with a high expression of MHC class I antigen after sorting by using flow cytometric techniques. Thus, the phenotype defines these cells as LD cells that are lineage-negative, CD71-negative, MHC class I bright. This is the same population of HSCs that Ogata et al. (1) showed to be wheat germ agglutinin-positive, Thy-1low, Sca-1+, and c-kit-negative or low.
SCT.
Recipient mice, 8–10 weeks old, were given 7.5 Gy (for BALB/c) or 9.5 Gy [for (B6 × C3H) F1 or B6] total body irradiation (137Cs irradiation, 0.75 Gy/min) and reconstituted i.v. by HSCs purified as mentioned above. There were three transplantation groups: fully allogeneic, B6 → BALB/c; hemiallogeneic, B6, Ly-5.1 → (B6 × C3H) F1; congeneic, B6 Ly-5.1 → B6 Ly-5.2. A competitive repopulation strategy was used to reconstitute lethally irradiated recipients with a few highly purified HSCs (10 up to 1,000 with MHC-matched donors; 100, up to 10,000 in hemiallogeneic donors; and 10,000, up to 1 × 105 in fully allogeneic donors). Two serial syngeneic bone marrow transplantation (BMT) of recipient mice was performed to obtain compromised cells needed to make possible long term evaluations in this competitive repopulation strategy. The compromised cells (treated with Thy-1.2 plus immunomagnetic beads in vitro to deplete T cells) supported survival of the lethally irradiated recipient beyond the otherwise limiting irradiation crisis.
Bone Transplantation.
BM was thoroughly flushed out of tibias or femurs of donor B6 mice. Fragments of bones then were transplanted under the kidney capsule of recipients 1 day after total body irradiation.
Stromal Cell Transplantation.
A primary marrow stromal cell population was obtained by culturing bones and their marrow from donor B6 mice at 37°C with 5% C02. Nonadherent cells were discarded each time while changing media. Adherent cells had been expanded for at least three passages in vitro before transplantation. Adherent cells (3 × 105) were injected i.v. together with HSCs in (B6 → BALB/c) SCT.
Thymectomy of Recipient Mice.
Some of the BALB/c recipients were thymectomized when they were 3 to 4 weeks old.
Preconditioning of Recipients with Granulocyte Colony-Stimulating Factor (G-CSF).
G-CSF (Amgen Biologicals, Thousand Oaks, CA) had been injected s.c. 6 μg/day per recipient for 5 consecutive days before performing a fully allogeneic SCT.
Assay of Chimerism.
Single cell suspensions of spleen cells from chimeric mice 4 months post-BMT were incubated with FITC-conjugated antibodies against donor or recipient phenotype, and subpopulations of donor cells (Kb, or Ly-5.1-positive) and recipient cells (Kd, or Ly-5.2-positive) were quantified by using flow cytometric analysis. Cell surface phenotypes for each lineage also were analyzed at the same time.
RESULTS
SCT.
The percentage of HSCs (LD, lineage-negative, CD71−, H-2 class Ibright) among total nucleated BM cells from 5-FU-treated donors was 0.4 ± 0.2% (mean ± SD, n = 7). The percentage of such HSCs would be 0.04% for those donors without 5-FU treatment because a single i.v. injection of 5-FU (150 mg/kg) usually depletes the number of BM nucleated cells by 90%. Such HSCs have been shown to produce reconstituting engraftment in male to female SCT even when a single HSC is used for the reconstruction (1).
Table 1 shows the chimerism of fully allogeneic, hemiallogeneic, and congeneic SCT. In fully allogeneic SCT, highly purified HSCs from 5-FU-treated B6 donors were transplanted into supralethally irradiated BALB/c recipients. Four months after transplantation, five of six of the chimeras transplanted with 1 × 105 HSCs showed hematopoietic cell engraftment of donor origin. Cells of donor origin were demonstrable in every hematopoietic lineage (Fig. 1). Two of six (33%) chimeras transplanted with 5 × 104 cells also showed engraftment of donor cells. None of the recipients transplanted with 1 × 104 HSCs exhibited donor cell engraftment.
Table 1.
Chimerism of SCT
| SCT | HSCs, ×103 | Chimeras with donor engraftment*, % | Cell phenotype, %
|
|
|---|---|---|---|---|
| Donor origin | Recipient origin | |||
| B6 → BALB (fully allogeneic) | 100 | 5/6 (83) | 52.7 ± 10.8* | 48.0 ± 12.7 |
| 50 | 2/6 (33) | 33.8 ± 14.6* | 64.0 ± 11.4 | |
| 10 | 0/9 (0) | 2.3 ± 0.8 | 98.3 ± 2.2 | |
| B6 Ly-5.1 → (B6 × C3H) F1 (hemiallogeneic) | 10 | 7/7 (100) | 78.1 ± 7.9 | 24.1 ± 5.5 |
| 1 | 8/8 (100) | 52.2 ± 24.1* | 45.1 ± 24.9 | |
| 0.1 | 1/8 (13) | 6.0 ± 4.7 | 84.7 ± 3.6 | |
| B6 Ly-5.1 → B6 Ly-5.2 (congeneic) | 1 | 10/10 (100) | 89.2 ± 3.4 | 11.6 ± 1.6 |
| 0.1 | 12/12 (100) | 26.7 ± 5.6 | 64.9 ± 8.7 | |
| 0.01 | 1/12 (8) | 5.5 ± 4.3 | 93.1 ± 4.9 | |
Spleen cells of chimeras 4 months post-SCT were stained with FITC-conjugated anti-H-2 Kb or anti-Ly-5.1 for donor origin or with FITC-conjugated anti-H-2 Kd or anti-Ly-5.2 for recipient origin. Each group consisted of at least three recipients, and each experiment had been repeated at least once. Results are mean ± SD.
Only those chimeras with >10% of cells of donor origin were included in calculations of donor engraftment and those of mean ± SD.
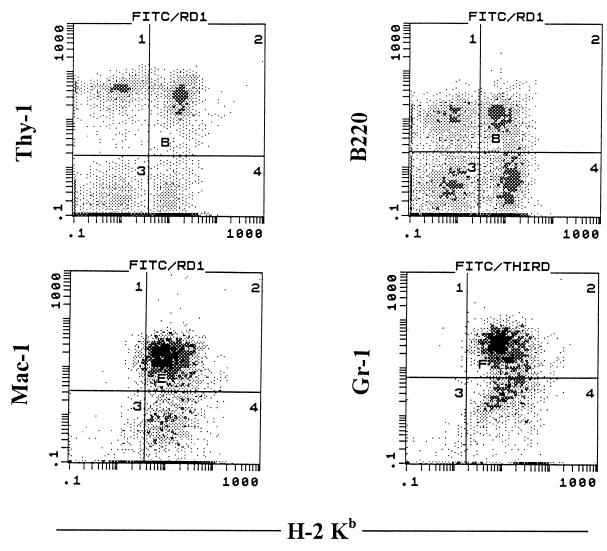
Figure 1.
Phenotypes of donor-derived cells in fully allogeneic SCT chimeras. One × 105 LD, lineage-negative, CD71−, H-2 class Ibright cells from 5-FU-treated B6 donor mice were transplanted into supralethally irradiated BALB/c recipients along with 4 × 105 compromised, T cell-purged BALB/c BM cells. Four months after SCT, spleen cells from these chimeras were stained with anti-H-2 Kb+ mAbs against Thy-1, B220, Mac-1, or Gr-1.
In hemiallogeneic SCT, HSCs from 5-FU-treated B6 Ly-5.1 donors were transplanted into (B6 × C3H) F1 mice. As shown in Table 1, SCT with 1,000 HSCs yielded a chimerism that involved >52.2% of donor cells. Cells of donor origin were demonstrable in every hematopoietic lineage (data not shown). SCT with 100 HSCs across this histocompatibility barrier showed only a very low percentage of cells of donor origin (6%).
Congeneic SCT had been carried out by using B6 mice that are congeneic for different alleles of the Ly-5 antigen. Highly purified HSCs from 5-FU-treated B6 Ly-5.1 mice were transplanted into 9.5-Gy-irradiated B6 Ly-5.2 recipients. All transplants showed donor engraftment after SCT with only 100 HSCs (Table 1). The percentages of cells of donor origin in every lineage of hematopoietic cells in the transplantations with 1,000 HSCs were comparable with the percentages of donor cells present in chimeras prepared as T cell purged BMTs (Table 2).
Table 2.
Lineages of cells of donor origin from congeneic chimeras
| Ly-5.1+* | Thy-1+† | B220+† | Mac-1+‡ | Gr-1+(c) | |
|---|---|---|---|---|---|
| SCT | 89.2 ± 3.4 | 52.9 ± 2.3 | 33.0 ± 1.7 | 45.2 ± 7.4 | 53.9 ± 5.8 |
| BMT | 91.6 ± 7.8 | 50.4 ± 9.3 | 31.7 ± 2.9 | 47.7 ± 5.4 | 56.4 ± 10.8 |
Expression of Ly-5.1+ spleen cells for markers of T cells, B cells, macrophages, and granular cells from congeneic Ly-5.1 → Ly-5.2 chimeras 4 months after SCT with 1 × 103 LD, lineage-negative, CD71−, H-2 class Ibright HSCs from 5-FU-treated donor mice or BMT with T cell-purged marrow cells. Results are mean ± SD.
Ly-5.1+ cells are those of donor origin.
Percentages of cells grated by lymphoid cell size.
Percentages of cells gated by myeloid/granular cell size.
Cotransplantation of Marrow Stromal Cells with HSCs in Allogeneic SCT.
Marrow stromal cells, either a primary culture or as contained in bone fragments, have been used in fully allogeneic SCT to investigate influence of the stromal cells to enhance engraftment of HSCs across MHC barriers. No cells of donor origin were detected in SCT with 1 × 104 HSCs plus either 3 × 105 primary marrow stromal cells, or bone transplantation simultaneous with the SCT (Table 3).
Table 3.
Effect of cotransplantation of marrow stromal cells with HSCs on allogeneic SCT
| Marrow stromal cells | H-2 Kb+ cells, % | H-2 Kd+ cells, % |
|---|---|---|
| None | 2.6 ± 1.0 | 96.7 ± 2.7 |
| Primary culture | 4.5 ± 1.6 | 95.7 ± 2.8 |
| Bone transplantation | 2.9 ± 0.9 | 98.2 ± 1.3 |
Spleen cells of recipient BALB/c mice transplanted with 1 × 104 LD, lineage-negative, CD71−, H-2 class Ibright HSCs from 5-FU-treated B6 donors with or without marrow stromal cells from the donor strain 4 months post-SCT were stained with FITC-conjugated H-2 Kb (donor phenotype) or FITC-conjugated H-2 Kd (recipient phenotype). Each experimental group consisted of at least three recipients. Results are mean ± SD.
Elimination of Recipient Resistance by Thymectomy or G-CSF.
We attempted to eliminate resistance of recipients either by using thymectomy or injecting G-CSF before SCT. As shown in Table 4, lethally irradiated BALB/c recipients transplanted with 1.5 × 105 LD, lineage-negative, CD71− HSCs from 5-FU-treated B6 donors did not produce reconstitution with donor cells. However, when recipient mice were preconditioned by thymectomy when they were 3–4 weeks old, three of seven (43%) showed engraftment of donor cells. Preconditioning BALB/c recipient mice by s.c. injection of G-CSF also enhanced the engraftment of donor HSCs (Table 4).
Table 4.
Effect of preconditioning recipient mice before SCT on engraftment of fully allogeneic HSCs
| Preconditioning | H-2 Kb+ cells, % | H-2 Kd+ cells, % |
|---|---|---|
| None | 3.5 ± 1.2 | 97.6 ± 1.7 |
| Thymectomy | 27.6 ± 12.3 | 59.7 ± 18.7 |
| G-CSF injection | 29.4 ± 10.6 | 67.5 ± 16.9 |
Spleen cells of recipient BALB/c mice transplanted with 1.5 × 105 HSCs (LD, lineage-negative, CD71−) from 5-FU-treated B6 donors were stained with FITC-conjugated H-2 Kb (donor type) or FITC-conjugated H-2 Kd (recipient type) 4 months post-SCT. Thymectomy was done with when recipients were 3 to 4 weeks old. G-CSF was given s.c. 6 μg/day/recipient for 5 consecutive days before SCT. Each experimental group consisted of at least three mice. Results are mean ± SD.
DISCUSSION
BMT has been used since 1968 to correct and often to cure children and adults suffering from as many as 75 different and otherwise highly lethal diseases (11–14). Recently, purification and transplantation of HSCs have been investigated extensively. SCT induces much less graft-vs.-host diseases than does the usual allogeneic BMT. This may be a real advantage because graft-vs.-host disease compromises marrow transplantation and limits its use to lethal or highly morbid diseases and has inhibited use of such cellular engineering for less morbid but distressing diseases such as autoimmunities. However, HSCs used alone in SCT could not engraft lethally irradiated fully allogeneic recipients (8–10). Because of the success of mismatched BMT and the failure of mismatched SCT, a population of cell(s) resident with the BM, but absent in the stem cell preparations, have been investigated as facilitators of successful mismatched BMT. Several studies have investigated this hypothesis, and several kinds of facilitating cells have been described (9, 10).
In the previous study by Ogata et al. (1), it was shown that highly purified marrow HSC (as few as 1–4 cells) can repopulate cells in every lineage of hematopoietic cells in male to female syngeneic transplantation. In the present study, we used the HSC purification technique used by Ogata et al. to investigate whether an increase in the number of well defined, highly purified HSCs alone without any facilitating cells can repopulate lethally irradiated, fully allogeneic recipient mice. We hypothesized that if donor HSCs alone can repopulate fully allogeneic recipients, engraftment of cells of donor origin might be achieved simply by increasing the number of allogeneic HSCs transplanted in SCT. Our results showed that by transplanting 1 × 105 LD, lineage-negative, CD71−, H-2 class Ibright HSCs from 5-FU-treated B6 donor mice into lethally irradiated, fully allogeneic BALB/c recipients, successful long term engraftment is achieved without cotransplantation of any facilitating cells. A decreased number of 1 × 104 HSCs from 5-FU-treated B6 mice, however, failed to engraft allogeneic BALB/c recipients, although 1 × 103 HSCs from 5-FU-treated B6 Ly-5.1 donors can readily repopulate every lineage of hematopoietic cells in hemiallogeneic (B6 × C3H) F1 and in congeneic B6 Ly-5.2 recipients. Our findings also show that as long as a sufficient number of HSCs is transplanted, long term engraftment can be achieved in fully allogeneic SCT. Facilitating cell(s), although possibly helpful (9, 10), are not a necessity in allogeneic SCT.
Ishida et al. (15) reported that MHC-matched stromal cells present within the BM may be essential to provide support of hematopoietic cells in G0 stage, which, together with stromal cells from donor, can successfully be used to treat in many manifestations of disease in MRL/lpr mice. In these mice, transplantation of BM is effective soon after transplantation, but the marrow graft is regularly lost unless stromal cells of a genetically similar donor also are used. Transplantation of stromal cells plus HSC from BM produce sustained remissions of autoimmune diseases in MRL/lpr mice. Bone transplants also were helpful and could be used as the source of such stromal cells. When bone transplants were used along with BMT in MRL/lpr mice, these dual transplants achieved long term remissions and apparent cures of all manifestations of disease in the MRL/lpr mice. We used a similar strategy in our present experiments to determine whether marrow stromal cells might facilitate engraftment of HSCs in allogeneic SCT across the entire MHC transplantation barrier. We found that marrow stromal cells, either from primary cultures or from bones of donor strain, did not facilitate engraftment of highly purified HSCs in fully allogeneic SCT.
We also investigated whether engraftment of allogeneic HSC can be enhanced by eliminating potential resistance of recipient mice. Data already published have shown that donor T cells can enhance the engraftment of allogeneic SCT or BMT (16, 17). From 1 to 3% of residual host spleen T cells remain after irradiation in usual BMT (data not shown). These residual T cells or T cells derived from residual HSC after SCT have been thought possibly to inhibit engraftment of donor HSC. Therefore, we attempted to eliminate recipient T cells by thymectomizing recipients 8–10 weeks old; engraftment of donor cells could be observed 4 months after SCT in the thymectomized recipients but not in those animals that had been sham-thymectomized. We also tried to decrease residual recipient HSC number by injecting G-CSF into recipients before allogeneic SCT. G-CSF has been used to mobilize HSCs to the blood for peripheral blood stem cell collection (18–20). Our results showed that although 1.5 × 105 LD, lineage-negative, CD71− failed to repopulate fully allogeneic recipients, engraftment of cells of donor origin was demonstrable in fully allogeneic recipients preconditioned with G-CSF for 5 consecutive days, transplanted with the same number of HSCs (1 × 105). In these experiments, we could not rule out the possibility that G-CSF injected on the day of SCT enhanced the differentiation of donor HSCs in the allogeneic environment.
We contend that we have shown herein that it is possible to engraft fully allogeneic recipient mice across the MHC barrier if a sufficiently large number of HSCs are transplanted. Such successful transplants are achieved without cotransplanting facilitating cells, and this is accomplished with evidence of long term hematopoietic repopulation. Such pure stem cell transplantations are achieved with high frequency. Following this lead, which, for now, is a phenomenon in mice, may enable us to prevent and possibly cure diseases simply by SCT across multimajor MHC plus multiminor histocompatibility barriers.
It already has been possible to prevent expression of autoimmune disease across MHC barriers by BMT and in some models to prevent or treat autoimmune diseases by BM plus stromal cells or bone grafts (15, 21). It now will be of great interest to determine whether we can exploit the present results to prevent and/or treat autoimmune diseases by transplantation of purified populations of HSC.
Acknowledgments
This work was supported by National Institute of Aging Grant 05628-13 and Pediatric Cancer Foundation to Children’s Research Institute, All Children’s Hospital, St. Petersburg, FL. We thank Ms. Tazim Verjee for her assistance in the preparation of the manuscript.
ABBREVIATIONS
- MHC
major histocompatibility complex
- HSC
hematopoietic stem cell
- 5-FU
5-fluorouracil
- SCT
stem cell transplantation
- LD
low density
- G-CSF
granulocyte colony-stimulating factor
- BM
bone marrow
- BMT
bone marrow transplantation
- FITC
fluorescein isothiocyanate
References
- 1.Ogata H, Bradley W G, Inaba M, Ogata N, Ikehara S, Good R A. Proc Natl Acad Sci USA. 1995;92:5945–5949. doi: 10.1073/pnas.92.13.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih J P, Ogawa M. Blood. 1993;81:1155–1160. [PubMed] [Google Scholar]
- 3.Spangrude G J, Smith L, Uchida N, Ikuta K, Heimfeld S, Freidman J, Weissman I L. Blood. 1991;78:1395–1402. [PubMed] [Google Scholar]
- 4.Harrison D E, Atle C M, Lerner C. Proc Natl Acad Sci USA. 1988;85:822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snodgrass R, Keller G. EMBO J. 1987;6:3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 7.Doi H, Inaba M, Yamamoto Y, Taketani S, Mori S-I, Sugihara A, Ogata H, Toki J, Hisha H, Inaba K, Sogo S, Adachi M, Matruda T, Good R A, Ikehara S. Proc Natl Acad Sci USA. 1997;94:2513–2517. doi: 10.1073/pnas.94.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Badri N S, Good R A. Proc Natl Acad Sci USA. 1993;90:6681–6685. doi: 10.1073/pnas.90.14.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman C L, Colson Y L, Wren S M, Watkins S, Simmons R L, Ildstad S T. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 10.El-Badri, N. S., Wang, B.-Y., Cherry & Good, R. A. (1998) J. Exp. Hematol., in press. [PubMed]
- 11.Gatti R A, Meuwissen H J, Allen H D, Hong R, Good R A. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 12.Good R A. Hosp Pract. 1969;4:41–47. [Google Scholar]
- 13.Good R A, Gatti R A, Hong R, Meuwissen H J. Exp Hematol. 1969;19:4–10. [Google Scholar]
- 14.Good R A. J Am Med Assoc. 1970;214:1289–1300. [Google Scholar]
- 15.Ishida T, Inaba M, Hisha H, Sugiura K, Adachi Y, Nagata N, Ogawa R, Good R A, Ikehara S. J Immunol. 1994;152:3119–3127. [PubMed] [Google Scholar]
- 16.Wang B, Onoé K, Good R A. In: Bone Marrow Transplantation: Foundations for the 21st Century. Sackstein R, Janssen W E, Elfenbein G J, editors. New York: New York Academy of Sciences; 1995. p. 379. [Google Scholar]
- 17.Arase-Fukushi N, Arase H, Wang B, Hirano M, Ogasawara K, Good R A, Onoé K. Microbiol Immunol. 1993;37:833–894. doi: 10.1111/j.1348-0421.1993.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 18.Ossenkoppele G J, Jonkhoff A R, Huijgens P C, Nauta J J, van der Hem K G, Grager A M, Langenhuijsen M M. Bone Marrow Transplant. 1994;13:37–41. [PubMed] [Google Scholar]
- 19.Suzue T, Kawano Y, Takaue Y, Kuroda Y. J Exp Hematol. 1994;22:888–892. [PubMed] [Google Scholar]
- 20.Bregni M, Siena S, Bonadonna G, Gianni A M. Eur J Cancer. 1994;30:235–238. doi: 10.1016/0959-8049(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang, B., Cherry, El-Badri, N. S. & Good, R. A. (1998) Proc. Natl. Acad. Sci. USA, in press.