Abstract
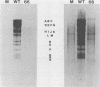
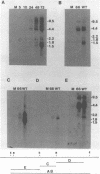
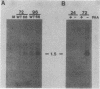
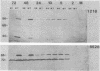
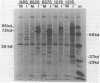
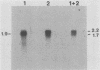
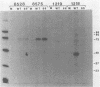
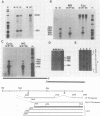
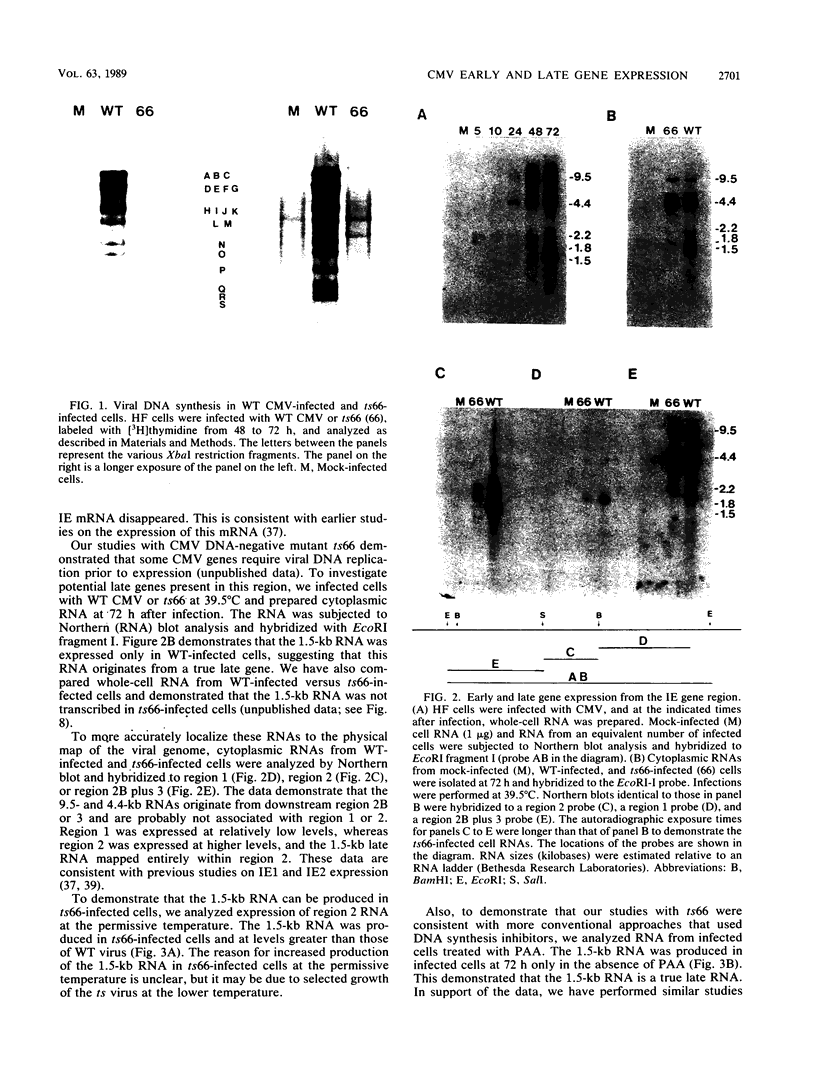
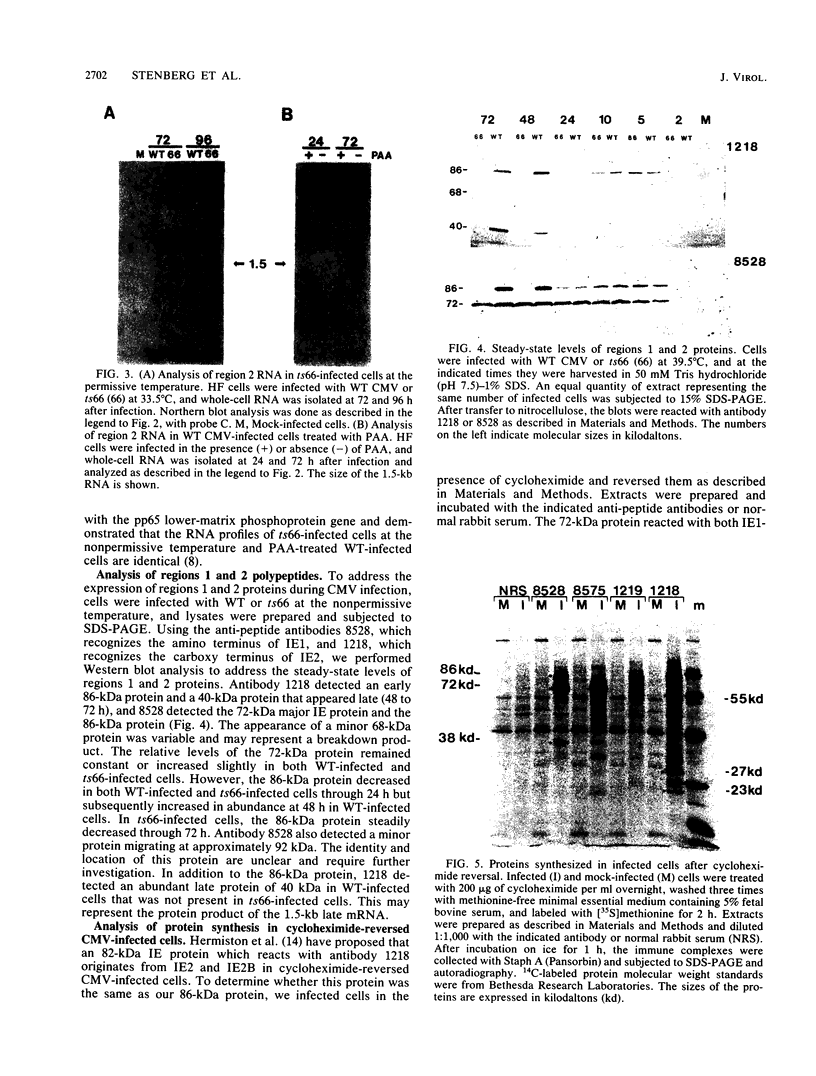
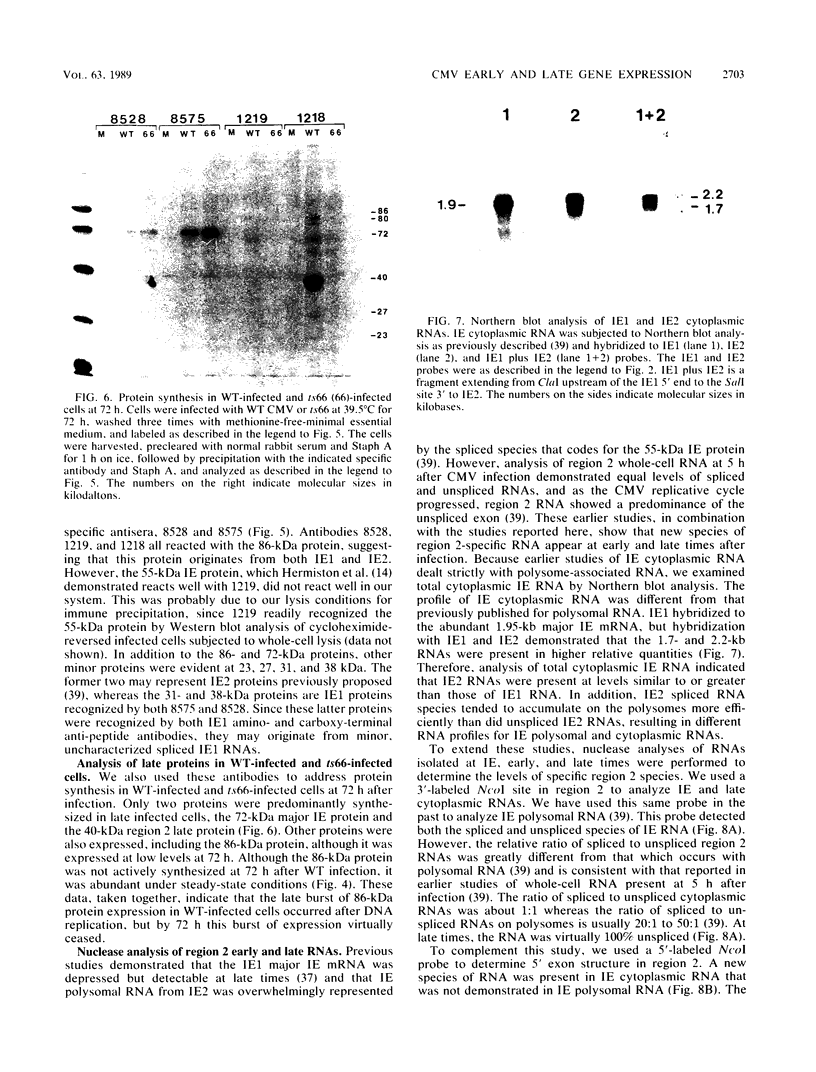
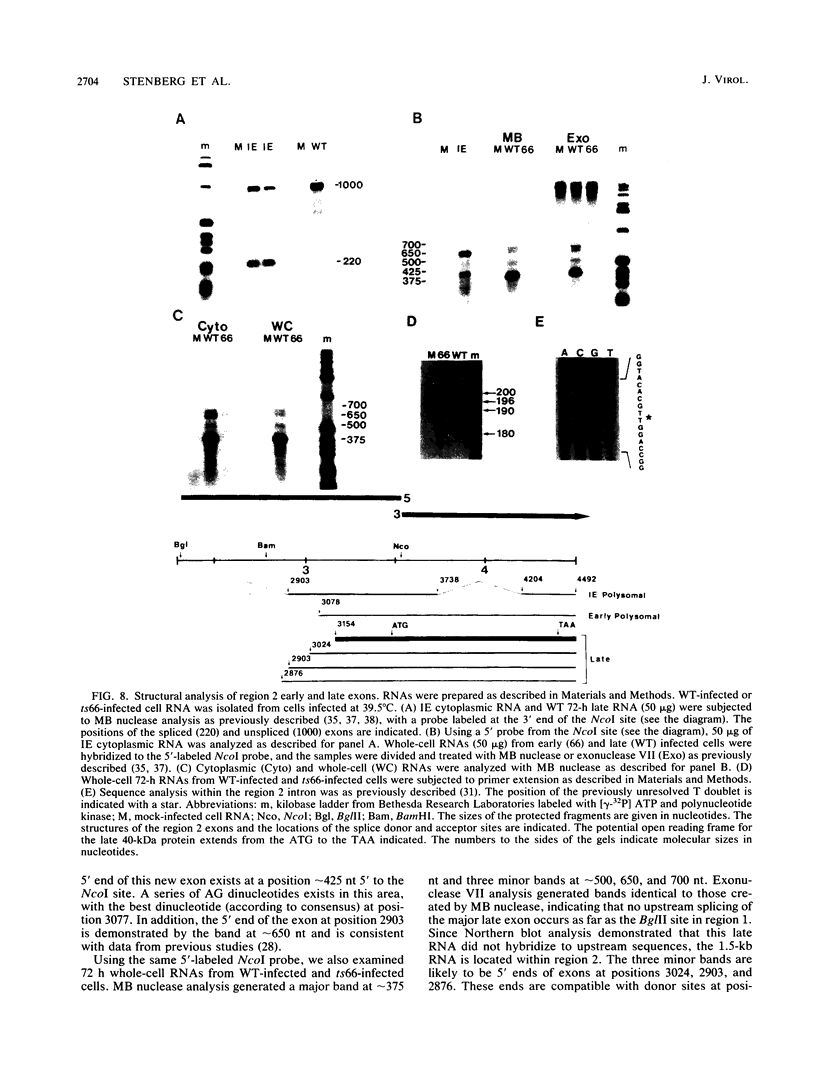
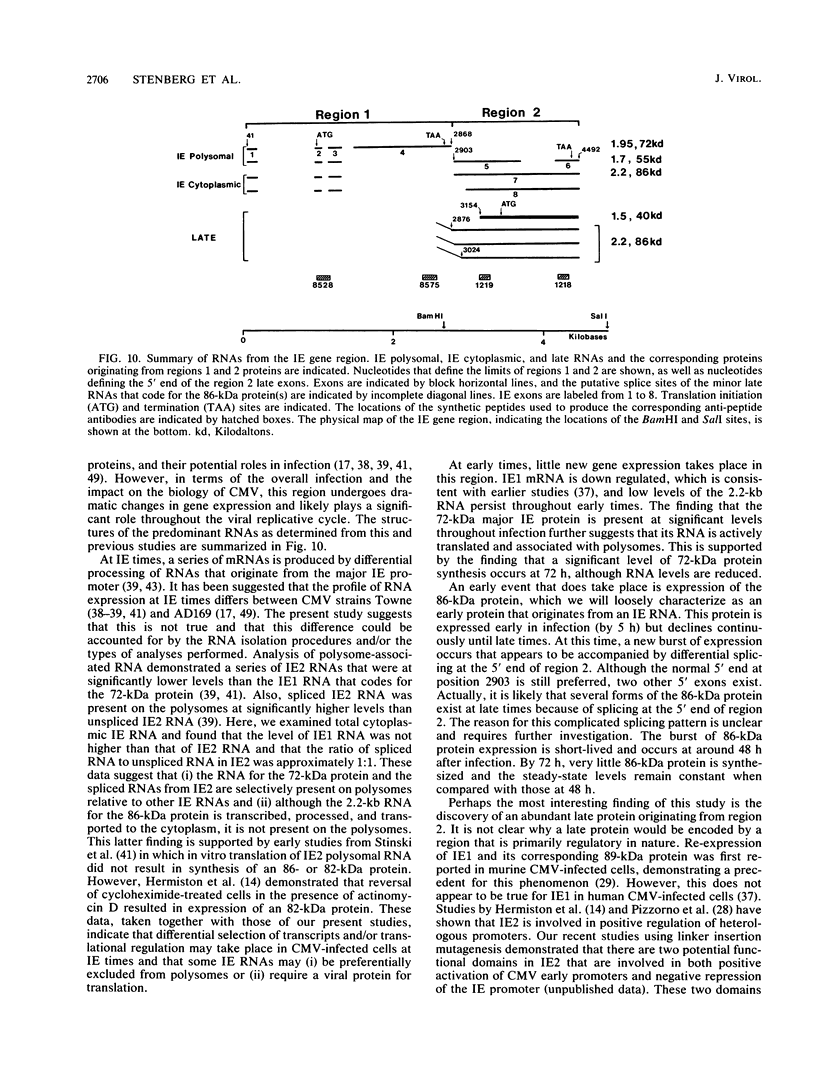
Expression of RNA and protein from the human cytomegalovirus immediate-early (IE) gene region (map units 0.732 to 0.751) was analyzed at early and late times after infection. The level of RNA present at late times (48 to 72 h after infection) was significantly higher than that present at IE times (5 h after infection). The profile of IE RNA in the cytoplasm of infected cells was different from that previously reported on polysomes (R. M. Stenberg, P. R. Witte, and M. F. Stinski, J. Virol. 56:665-675, 1985). The data indicate that the 1.95-kilobase (kb) major IE region 1 mRNA, which codes for the 72-kilodalton (kDa) protein, and the 1.7-kb IE region 2 (IE2) spliced mRNA, which codes for the IE2 55-kDa protein, may be preferentially associated with polysomes. However, the IE2 2.2-kb unspliced mRNA, which codes for an 86-kDa protein, may be preferentially excluded. This RNA was abundant in the cytoplasm under IE conditions but was not present on polysomes in significant quantities. This indicates that IE gene products may be involved in translational control of cytomegalovirus RNA. At late times, new transcription takes place within region 2. A 1.5-kb RNA is transcribed from a late promoter in region 2 that apparently does not function in cells infected with DNA-negative mutant ts66. These results demonstrate that the IE gene region is transcribed throughout infection and that multiple levels of regulation exist.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Gibson W. Location, transcript analysis, and partial nucleotide sequence of the cytomegalovirus gene encoding an early DNA-binding protein with similarities to ICP8 of herpes simplex virus type 1. J Virol. 1988 Apr;62(4):1364–1372. doi: 10.1128/jvi.62.4.1364-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Huang E. S. Nucleotide sequence of a human cytomegalovirus DNA fragment encoding a 67-kilodalton phosphorylated viral protein. J Virol. 1985 Oct;56(1):7–11. doi: 10.1128/jvi.56.1.7-11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Schmidt C. A., Kaplan A. S. Patterns of transcription of human cytomegalovirus in permissively infected cells. J Virol. 1980 Aug;35(2):277–286. doi: 10.1128/jvi.35.2.277-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depto A. S., Stenberg R. M. Regulated expression of the human cytomegalovirus pp65 gene: octamer sequence in the promoter is required for activation by viral gene products. J Virol. 1989 Mar;63(3):1232–1238. doi: 10.1128/jvi.63.3.1232-1238.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Nelson J. A., Walker L., Oldstone M. B. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: a means for graft rejection and immunosuppression. J Virol. 1988 Jan;62(1):100–105. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins W. F., Stinski M. F. Expression of a human cytomegalovirus late gene is posttranscriptionally regulated by a 3'-end-processing event occurring exclusively late after infection. Mol Cell Biol. 1986 Dec;6(12):4202–4213. doi: 10.1128/mcb.6.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Wilkinson G. W. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987 Feb;7(1):17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Jahn G., Bürkle A., Freese U. K., Fleckenstein B., zur Hausen H. Genomic localization, sequence analysis, and transcription of the putative human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):119–124. doi: 10.1128/jvi.61.1.119-124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston T. W., Malone C. L., Witte P. R., Stinski M. F. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J Virol. 1987 Oct;61(10):3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson N. I., Sondermeyer R. T., Tocci M. J. Organization and expression of the major genes from the long inverted repeat of the human cytomegalovirus genome. Virology. 1986 Nov;155(1):160–171. doi: 10.1016/0042-6822(86)90176-5. [DOI] [PubMed] [Google Scholar]
- Hutchinson N. I., Tocci M. J. Characterization of a major early gene from the human cytomegalovirus long inverted repeat; predicted amino acid sequence of a 30-kDa protein encoded by the 1.2-kb mRNA. Virology. 1986 Nov;155(1):172–182. doi: 10.1016/0042-6822(86)90177-7. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble G. W., McCormick A. L., Pereira L., Mocarski E. S. A cytomegalovirus protein with properties of herpes simplex virus ICP8: partial purification of the polypeptide and map position of the gene. J Virol. 1987 Oct;61(10):3143–3151. doi: 10.1128/jvi.61.10.3143-3151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Preddy E., Barrell B. G. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology. 1988 Jul;165(1):151–164. doi: 10.1016/0042-6822(88)90668-x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Staprans S. I., Spector D. H. Analysis of the major transcripts encoded by the long repeat of human cytomegalovirus strain AD169. J Virol. 1985 Mar;53(3):711–718. doi: 10.1128/jvi.53.3.711-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Fibi M. R., Keil G. M., Koszinowski U. H. Late-phase expression of a murine cytomegalovirus immediate-early antigen recognized by cytolytic T lymphocytes. J Virol. 1986 Dec;60(3):1125–1129. doi: 10.1128/jvi.60.3.1125-1129.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Stinski M. F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985 Dec;56(3):676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R., Spector D., Pizer L. Modulation of herpes simplex virus replication in adenovirus transformed cells. J Gen Virol. 1979 Aug;44(2):297–309. doi: 10.1099/0022-1317-44-2-297. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]