Abstract
Herpes simplex virus type 1 establishes latent infection in trigeminal ganglia of mice infected via the eye. A family of three colinear viral transcripts (LATs), 2.0, 1.5, and 1.45 kb, is present in latently infected ganglia. To characterize these LATs, lambda gt10 cDNA libraries were constructed with RNAs isolated from the trigeminal ganglia of latently infected mice. A series of recombinant bacteriophage were isolated containing cDNA inserts covering 1.7 kb of the 2.0-kb LAT. Splice junctions of the smaller LATs and the 3' end of the 2.0-kb LAT were identified by sequence analysis of RNA polymerase chain reaction products. No splice acceptor site, which does not support the hypotheses that the 2.0-kb LAT is an intron. However, the data are consistent with the possibility of a short leader sequence or multiple LAT transcription start sites. To generate the smaller 1.5- and 1.45-kb LATs, there is a 559-nucleotide intron spliced from the 2.0-kb LAT in strain F and a 556-nucleotide intron in strain 17+. The nucleotide sequences at the 5' and 3' ends of these introns are characteristic of spliced transcripts from eukaryotic protein-encoding genes, with one significant difference; i.e., the 5' end of the LAT intron is GC instead of the consensus sequence GT. This splice donor sequence is conserved in herpes simplex virus type 1 strains F, 17+, and KOS. Processing of the 2.0-kb LAT to form the spliced LATs preserves two open reading frames (ORFs) at the 3' end of the LATs; no new ORFs are created. Splicing of the LATs positions a 276-nucleotide leader sequence close to these ORFs and removes an intron that inhibits their translation in vitro. The novel 5' structure of the intron within the 2.0-kb LAT may be part of a control mechanism for transcription processing that results in splicing of the LATs only in sensory neurons during latent infection and reactivation but not during the viral replication cycle.
Full text
PDF





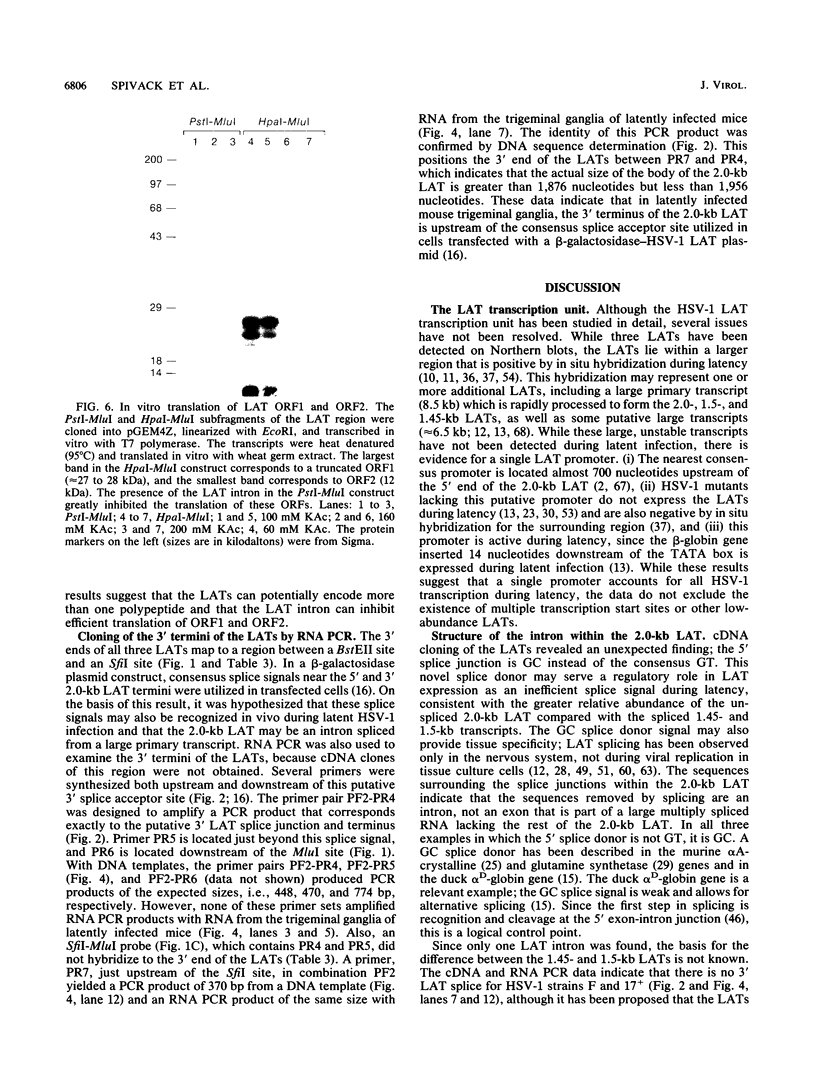




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Pettersson U. Sequence analysis of adenovirus DNA: complete nucleotide sequence of the spliced 5' noncoding region of adenovirus 2 hexon messenger RNA. Cell. 1979 Apr;16(4):841–850. doi: 10.1016/0092-8674(79)90099-0. [DOI] [PubMed] [Google Scholar]
- Batchelor A. H., O'Hare P. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990 Jul;64(7):3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Block T. M., Spivack J. G., Steiner I., Deshmane S., McIntosh M. T., Lirette R. P., Fraser N. W. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J Virol. 1990 Jul;64(7):3417–3426. doi: 10.1128/jvi.64.7.3417-3426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Shimada T., Moulton A. D., Cline A., Humphries R. K., Maizel J., Nienhuis A. W. The functional human dihydrofolate reductase gene. J Biol Chem. 1984 Mar 25;259(6):3933–3943. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi-Rao G. B., Goodart S. A., Hecht L. M., Rochford R., Rice M. K., Wagner E. K. Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol. 1991 May;65(5):2179–2190. doi: 10.1128/jvi.65.5.2179-2190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A. T., Sederati F., Devi-Rao G., Flanagan W. M., Farrell M. J., Stevens J. G., Wagner E. K., Feldman L. T. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J Virol. 1989 Sep;63(9):3844–3851. doi: 10.1128/jvi.63.9.3844-3851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C., Pizer L. I., Wilcox C. L. An antigen encoded by the latency-associated transcript in neuronal cell cultures latently infected with herpes simplex virus type 1. J Virol. 1991 May;65(5):2724–2727. doi: 10.1128/jvi.65.5.2724-2727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbil C., Niessing J. The primary structure of the duck alpha D-globin gene: an unusual 5' splice junction sequence. EMBO J. 1983;2(8):1339–1343. doi: 10.1002/j.1460-2075.1983.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. J., Dobson A. T., Feldman L. T. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S., Preston C. M., Smiley J. R., Summers W. C., Summers W. P. Utilization of internal AUG codons for initiation of protein synthesis directed by mRNAs from normal and mutant genes encoding herpes simplex virus-specified thymidine kinase. J Virol. 1985 Nov;56(2):512–519. doi: 10.1128/jvi.56.2.512-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R. T., Stevens J. G., Dissette V. B., Wagner E. K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988 Sep;166(1):254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Caton A. J., Staudt L. M., Schwartz D., Gerhard W. A set of closely related antibodies dominates the primary antibody response to the antigenic site CB of the A/PR/8/34 influenza virus hemagglutinin. J Immunol. 1990 Oct 1;145(7):2312–2321. [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. F., Darnell J. E., Jr Mouse glutamine synthetase is encoded by a single gene that can be expressed in a localized fashion. J Mol Biol. 1989 Jul 5;208(1):45–56. doi: 10.1016/0022-2836(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue R. A., Kraner S. D., Goodman R. H., Mandel G. Neuron-specific expression of the rat brain type II sodium channel gene is directed by upstream regulatory elements. Neuron. 1990 Feb;4(2):223–231. doi: 10.1016/0896-6273(90)90097-y. [DOI] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Lirette R. P., Fraser N. W. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):125–132. doi: 10.1099/0022-1317-71-1-125. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Steiner I., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. A herpes simplex virus type 1 variant, deleted in the promoter region of the latency-associated transcripts, does not produce any detectable minor RNA species during latency in the mouse trigeminal ganglion. J Gen Virol. 1990 Apr;71(Pt 4):953–957. doi: 10.1099/0022-1317-71-4-953. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Butow R. A. Mobile introns and intron-encoded proteins. Science. 1989 Dec 1;246(4934):1106–1109. doi: 10.1126/science.2479980. [DOI] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Campbell M. E., Clements J. B. A tandemly reiterated DNA sequence in the long repeat region of herpes simplex virus type 1 found in close proximity to immediate-early mRNA 1. J Virol. 1984 Nov;52(2):715–718. doi: 10.1128/jvi.52.2.715-718.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 (HSV-1) latency-associated transcripts and transcripts affected by the deletion in avirulent mutant HFEM: evidence for a new class of HSV-1 genes. J Virol. 1988 Sep;62(9):3281–3287. doi: 10.1128/jvi.62.9.3281-3287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988 May;62(5):1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., O'Boyle D. R., 2nd, Fraser N. W. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J Virol. 1987 Oct;61(10):3288–3291. doi: 10.1128/jvi.61.10.3288-3291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., O'Boyle D. R., 2nd, Lavi E., Fraser N. W. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J Virol. 1988 Sep;62(9):3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Haarr L., Porter D. D., Cook M. L., Wagner E. K. Prominence of the herpes simplex virus latency-associated transcript in trigeminal ganglia from seropositive humans. J Infect Dis. 1988 Jul;158(1):117–123. doi: 10.1093/infdis/158.1.117. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Trousdale M. D., Steiner I., Spivack J. G., Deshmane S. L., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991 Dec;65(12):6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Preston C. M., Clements J. B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNA's. J Virol. 1979 Jul;31(1):42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Zwaagstra J., Ghiasi H. Sequence of the latency-related gene of herpes simplex virus type 1. Virology. 1989 Jan;168(1):168–172. doi: 10.1016/0042-6822(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Zwaagstra J. C., Ghiasi H., Slanina S. M., Nesburn A. B., Wheatley S. C., Lillycrop K., Wood J., Latchman D. S., Patel K., Wechsler S. L. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990 Oct;64(10):5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaagstra J., Ghiasi H., Nesburn A. B., Wechsler S. L. In vitro promoter activity associated with the latency-associated transcript gene of herpes simplex virus type 1. J Gen Virol. 1989 Aug;70(Pt 8):2163–2169. doi: 10.1099/0022-1317-70-8-2163. [DOI] [PubMed] [Google Scholar]







