Abstract
In fetal sheep, circulating androgens influence fetal stress responsiveness and timing of parturition. Nevertheless, little is known about the presence and development of androgen receptors in the fetal brain. The present study was undertaken to test the hypothesis that expression of androgen receptor occurs in fetal brain and pituitary, and that the abundance of the AR is ontogenetically regulated. We isolated mRNA from pituitary, hypothalamus, hippocampus, and brainstem in fetal sheep that were 80, 100, 120, 130, 145 days gestation, and 1 and 7 days postnatal (n=4–5/group). Using real-time RT-PCR, we measured mRNA expression levels of the receptor in these brain regions and pituitary. In a separate study, we isolated protein from the same brain regions in fetal sheep that were 80 (n=3), 120 (n=4), and 145 (n=4) days. AR mRNA expression in hypothalamus increased in late gestation, starting at 145 days, and increasing progressively after birth. A trend of increasing AR protein in hypothalamus was not significant. AR mRNA expression in pituitary was elevated after 80 days gestation, but with no further increases or decreases in late gestation, while AR protein increased significantly at the end of gestation. In hippocampus and brainstem AR mRNA was constant throughout the latter half of gestation, and AR protein was below the sensitivity of our western blot assay. We conclude that the fetal brain and pituitary are target sites for circulating androgens or androgen precursors in fetal plasma, and we speculate that the increase in hypothalamic action of androgens immediately prior to birth might be integral to the timing of parturition.
Keywords: real-time PCR, sheep, parturition, neuroendocrinology, placenta, adrenal
In fetal sheep, there are several notable developmental changes in the neuroendocrine control mechanisms that prepare the fetus for postnatal life. We and others have reported maturational changes in the neuroendocrine components of the fetal hypothalamus-pituitary-adrenal axis; the development of this endocrine axis is important for the development of appropriate fetal responses to stress as well as the control of parturition in this species [1]. Late gestation is characterized by marked changes in the concentrations of several steroid hormones, including glucocorticoids, estrogens, and androgens [2–6]. We have recently reported developmental changes in the expression of glucocorticoid and mineralocorticoid receptors in fetal brainstem, hypothalamus, and pituitary [7]. For example, decreases in the pituitary expression of the glucocorticoid receptor coincides with decreased sensitivity of the fetal sheep to glucocorticoid negative feedback [8]. We have reported that androgen administration to fetal sheep results in altered stress responsiveness of the hypothalamus-pituitary-adrenal axis [9], and promotes premature parturition [10]. These observations have lead us to suspect that there might be an endogenous increase in the expression of androgen receptor in the fetal brain or pituitary at the end of gestation, influencing both stress responsiveness and the timing of parturition. Little is known about the development of the androgen receptor in the ovine fetal brain or pituitary. The present study is designed to test the hypothesis that expression of androgen receptor (AR) occurs in the ovine fetal brain and pituitary, and that the abundance of the AR is ontogenetically regulated.
Tissues used in this study were obtained from newborn (n=4) and 1 week old (n=4) lambs, and 24 fetal sheep of the following ages: 80 (n=5), 100 (n=4), 120 (n=4), 130 (n=4), 145 (n=4) days gestation. The animals were humanely euthanized using an overdose of sodium pentobarbital intravenously. Fetal brain and pituitary were rapidly isolated, dissected into relevant anatomical regions, snap-frozen in liquid nitrogen, and then stored at −80°C until studied. The medullary brainstem tissue was sectioned ~1 mm rostral to the obex and at the caudal medulla-rostral spinal cord border. Whole hippocampus was dissected bilaterally. Pituitary was removed in its entirety after removal of the diaphragma sella. Hypothalamus was removed as a single block of tissue, bounded on the rostral edge by the rostral edge of the optic chiasm, on the caudal side by the caudal edge of the median eminence, and on the sides by the edges of the median eminence. These experiments were approved by the University of Florida Institutional Animal Care and Use Committee.
Messenger RNA (mRNA) was isolated using Trizol® (Gibco, Invitrogen Corp., Carlsbad, CA), according to the manufacturer's instructions. After isolation, mRNA was stored in RNA Secure® (Ambion Corp., Austin, TX) at −80C until use. Total mRNA in each sample (4 µg) was converted to cDNA using a High Capacity cDNA Archive kit using methodology recommended by the kit manufacturer (Applied Biosystems, Foster City, CA). The newly synthesized cDNA, stored at −20C, was used for assay of mRNA for ovine Androgen Receptor by real-time polymerase chain reaction (PCR). Real-time PCR reactions were run using AmpliTaq Gold® DNA Polymerase (Applied Biosystems) and primers and probe (Geno-Mechanix, Alachua, FL) specifically designed using Primer Express software (Applied Biosystems). Probe for ovine Androgen Receptor (oAR) was labeled with 6-FAM in the 5’ position and TAMRA in the 3’ position. In each sample, 18S ribosomal RNA was also measured using real-time RT-PCR methodology, with probe, primers, and reagents purchased from Applied Biosystems. All mRNA abundances for oAR were normalized to the abundance of 18S rRNA, using the relative cycle threshold (ΔCt) method. All reactions were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems), using 100 ng cDNA, 100 nM primers, and 200 nM probe. Reactions were amplified using the following conditions: 48C for 30 minutes and 95C for 10 minutes, followed by 40 cycles of 95C for 15 seconds and 60C for 1 minute.
Sequences of primers and probe are reported in Table 1. oAR probe and primers were designed from oAR mRNA sequence accession number AF105713. The probe and primer set was designed using Primer Express 2.0 software from Applied Biosystems.
Table 1.
Ovine Androgen Receptor Primers and Probe
| Forward Primer | 5’-GCC CCT GAC CTG GTT TTC A-3’ |
| Reverse Primer | 5’-TTC GGA CAC ACT GGC TGT ACA-3’ |
| Probe | 5’-6FAM-TGA GTA CCG CAT GCA CAA GTC CCG-TAMRA-3’ |
For isolation of protein, tissue was homogenized in 5 volumes of boiling lysis buffer (1% SDS, 1.0 mM sodium orthovanadate, 10 mM Tris pH 7.4), boiled, centrifuged to remove particulates, aliquotted, then stored at −80C until assayed. The protein concentration of the supernatant was measured using a modified Bradford method (BioRad Co., San Rafael, CA) using bovine serum albumin (Sigma Chemical, St. Louis, MO) as the standard [11]. For analysis, aliquots were thawed on ice, boiled, electrophoresed with equal protein mass in each lane. Electrophoresis was performed using a Criterion gel and transfer apparatus (BioRad, San Rafael, CA) and precast 7.5% gels. The electrophoresed proteins were electroblotted onto nitrocellulose membranes (0.45 ìm pore size, BioRad). After transfer to the nitrocellulose membrane, the blot was probed with polyclonal antibody specific for AR overnight. AR antibody (N-20antibody, cat. no. sc-816, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was diluted 1:200 in antibody diluent (5% milk in PBS with 0.05% Tween 20). Membranes were visualized using goat-peroxidase conjugated anti-rabbit IgG (Sigma Chemical Co.) and ECL Plus reagent (Amersham, Arlington Heights, IL), and visualized using a BioRad Versadoc MP imager (BioRad). Quantity One densitometry software (BioRad) was utilized for blot analysis. Molecular weight was calibrated using Rainbow® molecular weight markers (Amersham Co.).
The expression of AR mRNA was normalized to 18S mRNA and reported graphically as the change relative to the mean concentration at 80 days gestation. The calculation of relative expression was performed using the ΔΔCt method, as described previously. Statistical analysis, on the other hand, was performed on the values of ΔCt, because these values are normally distributed [12]. In western blots, samples from all ages in a single brain region were analyzed in a single gel, so as to eliminate between-assay variation. Values of ΔCt (for mRNA) or optical density (for protein) were analyzed using one-way analysis of variance (SPSS 13.0, Chicago, IL), and pairwise comparisons of group means were performed using the Bonferroni test [13]. The null hypothesis (i.e. all groups are similar) was rejected when p < 0.05.
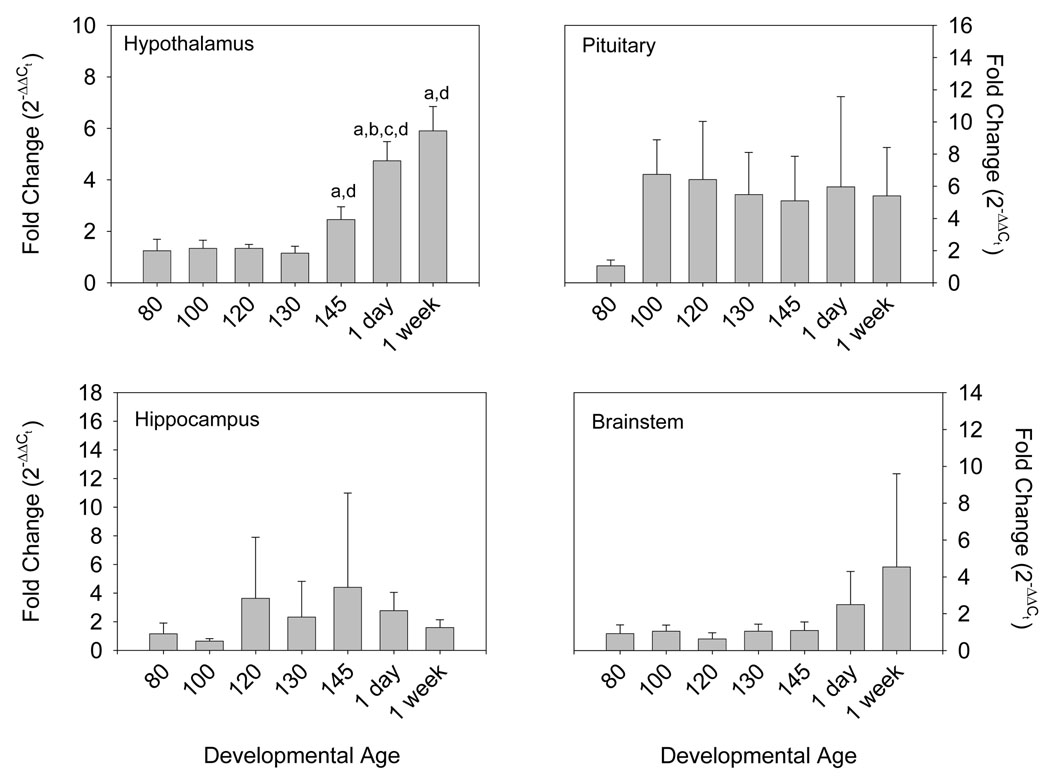
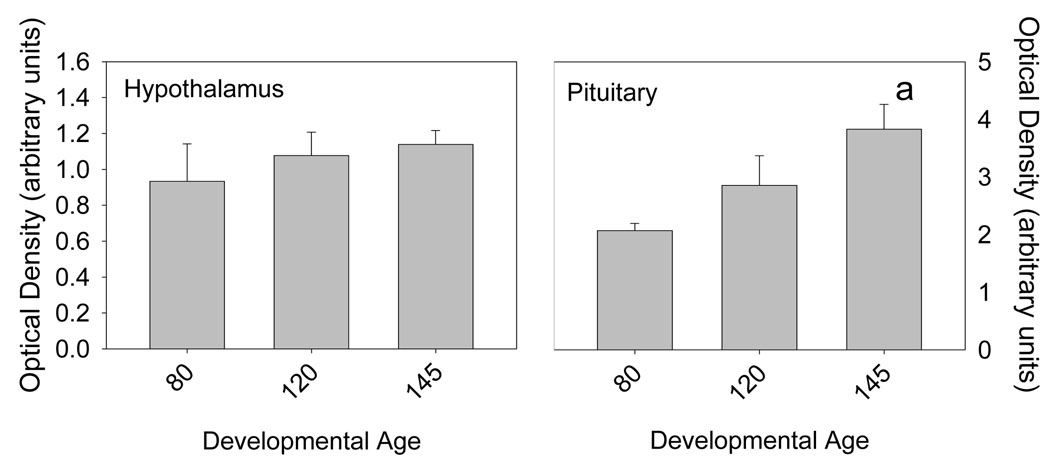
Androgen receptor mRNA was expressed in all of the tested tissues: hypothalamus, hippocampus, brainstem, and pituitary. There was a statistically significant ontogenetic pattern of AR expression in hypothalamus (p<0.001 by ANOVA, Figure 1, top left panel). Expression was low throughout most of the latter half of gestation (80–130 days gestation), then increased progressively starting at 145 days (p<0.05 by Bonferroni). AR protein, measured at 80, 120, and 145 days gestation, appeared to increase slightly throughout the latter half of gestation, although the apparent changes were not statistically significant. In pituitary (Figure 1, top right panel), there was a statistically significant (p<0.05 by ANOVA and Bonferroni) increase in AR expression after 80 days gestation. After 80 days, pituitary expression of AR mRNA was constant, although somewhat variable. AR protein in the pituitary increased significantly at 145 days gestation compared to earlier ages (Figure 2). In hippocampus (Figure 1, bottom left panel), expression of AR was variable, but there were no consistent or statistically significant (p=NS) changes as a function of developmental age. In brainstem (Figure 1, bottom right panel), there was an appearance of increased expression postnatally, although this was not statistically significant (p=NS). AR protein was not measurable in brainstem or hippocampus.
Figure 1.
Ovine Androgen Receptor mRNA abundance in hypothalamus (panel A), brainstem (panel B), hippocampus (panel C), and pituitary (panel D) from 80, 100, 120, 130, and 145 day gestation fetal sheep and 1 and 7 day postnatal lambs (n=4–5 per group). Data are expressed as fold change relative to 80 day fetal sheep. Data are represented as mean values ± 1 SEM. “a” represents statistically significant difference from 80 day gestation fetal sheep, “b” represents significant difference from 100 days, “c” represents significant difference from 120 days, and “d” represents significant difference from 130 day gestation fetal sheep.
Figure 2.
Immunoreactive ovine Androgen Receptor abundance in hypothalamus (left) and pituitary (right) in fetal sheep that are 80 (n=3), 120 (n=4), and 145 (n=4) days gestation. Data are represented as mean values ± 1 SEM. “a” represents statistically significant difference from 80 day gestation fetal sheep.
This is the first report of the ontogenetic pattern of expression of the androgen receptor in the developing fetus. Nevertheless, the results of the present study are consistent with previously reported studies from other laboratories. Previous reports have demonstrated androgen receptor at the protein and receptor binding levels in fetal hypothalamus, hippocampus, and pituitary of the developing rhesus macaque [14–17]. Androgen receptor binding activity in the rhesus macaque is increased in late gestation compared to mid-gestation [18].
Fetal development, homeostasis, and mechanisms controlling the timing of parturition are influenced by both circulating and locally-produced steroid hormones [19;20]. We and others have investigated the changes in hormone secretion and action, especially at or near the end of gestation, which prepare the fetus for extrauterine life [2–4;10;21;22]. For example, it is known that the placenta secretes increasing quantities of estrogen and androgen at the end of gestation and that, with a proportion that varies for each steroid, some of the steroid is secreted into the fetal blood [3;22;23]. In the sheep, for example, fetal plasma concentrations of estrone, estrone sulfate, androstenedione, testosterone, increase at the end of gestation [22;23]. The source of these steroids in late gestation is the placenta, although the target tissues in the fetus have not been clearly defined. We propose that the fetal brain and pituitary are important sites of both androgen and estrogen action, and that the action of these two classes of hormones combine to prepare the fetus for transition to neonatal life.
The presence and active regulation of androgen receptor in fetal hypothalamus (at the mRNA level) and pituitary (at the protein level) prior to the timing of normal parturition suggests an important physiological action of androgen at that time. The function of estrogen and androgen action in late gestation in the fetal brain is not clear. Prenatal treatment of fetal sheep with androgen does alter postnatal reproductive behavior and growth; however, these treatments are effective relatively early in gestation (60–90 days gestation) [24;25]. Nevertheless, it is possible that the increased expression of androgen receptor at 145 days gestation might be critical for pituitary gonadotrope maturation [26] or for the process of initiation of parturition and preparation for extrauterine life [10].
In summary, we report that the fetal hypothalamus, hippocampus, brainstem, and pituitary express androgen receptor, that the expression in the hypothalamus and pituitary are increased starting in late gestation. We conclude that the fetal brain and pituitary are target sites for circulating androgens or androgen precursors in fetal plasma, and we speculate that the increase in hypothalamic action of androgens immediately prior to birth might be integral to the timing of parturition.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Child Health and Human Development, HD42135 (to CEW). The authors thank Ms. Xiaoyang (Lisa) Fang for her expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Liggins GC. Initiation of spontaneous labor. Clin. Obstet. Gynecol. 1983;26:47–55. doi: 10.1097/00003081-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Rose JC, Macdonald AA, Heymann MA, Rudolph AM. Developmental aspects of the pituitary-adrenal axis response to hemorrhagic stress in lamb fetuses in utero. J. Clin. Invest. 1978;61:424–432. doi: 10.1172/JCI108953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsner CW, Magyar DM, Fridshal D, Eliot J, Glatz T, Klein AH, Nathanielsz PW, Buster JE. Time-trend analysis of plasma C-21 steroids in fetal and maternal sheep during the last 18 days of gestation. Endocrinology. 1980;107:801–808. doi: 10.1210/endo-107-3-801. [DOI] [PubMed] [Google Scholar]
- 4.Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107:1155–1159. doi: 10.1210/endo-107-1-155. [DOI] [PubMed] [Google Scholar]
- 5.Magyar DM, Elsner CW, Fridshal D, Eliot J, Klein A, Glatz T, Lowe KC, Nathanielsz PW, Buster JE. Time-trend analysis of plasma 11-desoxycorticosterone, corticosterone, cortisol, and aldosterone in fetal and maternal sheep during the last 18 days of gestation. J. Steroid Biochem. 1981;14:1091–1099. doi: 10.1016/0022-4731(81)90221-1. [DOI] [PubMed] [Google Scholar]
- 6.Thorburn GD, Nicol DH, Bassett JM, Shutt DA, Cox RI. Parturition in the goat and sheep: changes in corticosteroids, progesterone, oestrogens and prostaglandin F. J. Reprod. Fertil.Suppl. 1972;16 Suppl-84 [PubMed] [Google Scholar]
- 7.Keller-Wood M, Powers MJ, Gersting JA, Ali N, Wood CE. Genomic analysis of neuroendocrine development of fetal brain-pituitary-adrenal axis in late gestation. Physiol Genomics. 2006;24:218–224. doi: 10.1152/physiolgenomics.00176.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wood CE. Insensitivity of near-term fetal sheep to cortisol: Possible relation to the control of parturition. Endocrinology. 1988;122:1565–1572. doi: 10.1210/endo-122-4-1565. [DOI] [PubMed] [Google Scholar]
- 9.Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am. J. Physiol. 1997;272:R1128–R1134. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- 10.Wood CE, Saoud CJ. Influence of estradiol and androstenedione on ACTH. J. Soc. Gynecol. Investig. 1997;4:279–283. [PubMed] [Google Scholar]
- 11.Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J. Physiol. 2003;549:573–581. doi: 10.1113/jphysiol.2002.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. pp. 514–603. [Google Scholar]
- 14.Choate JV, Slayden OD, Resko JA. Immunocytochemical localization of androgen receptors in brains of developing and adult male rhesus monkeys. Endocrine. 1998;8:51–60. doi: 10.1385/ENDO:8:1:51. [DOI] [PubMed] [Google Scholar]
- 15.Pomerantz SM, Fox TO, Sholl SA, Vito CC, Goy RW. Androgen and estrogen receptors in fetal rhesus monkey brain and anterior pituitary. Endocrinology. 1985;116:83–89. doi: 10.1210/endo-116-1-83. [DOI] [PubMed] [Google Scholar]
- 16.Pomerantz SM, Sholl SA. Analysis of sex and regional differences in androgen receptors in fetal rhesus monkey brain. Developmental Brain Research. 1987;36:151–156. doi: 10.1016/0165-3806(87)90074-5. [DOI] [PubMed] [Google Scholar]
- 17.Sholl SA, Kim KL. Aromatase, 5-alpha-reductase, and androgen receptor levels in the fetal monkey brain during early development. Neuroendocrinology. 1990;52:94–98. doi: 10.1159/000125545. [DOI] [PubMed] [Google Scholar]
- 18.Handa RJ, Connolly PB, Resko JA. Ontogeny of cytosolic androgen receptors in the brain of the fetal rhesus monkey. Endocrinology. 1988;122:1890–1896. doi: 10.1210/endo-122-5-1890. [DOI] [PubMed] [Google Scholar]
- 19.Wood CE. Control of parturition in ruminants. J. Reprod. Fertil. Suppl. 1999;54:115–126. [PubMed] [Google Scholar]
- 20.Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J. Soc. Gynecol. Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Saoud CJ, Wood CE. Ontogeny and molecular weight of immunoreactive arginine vasopressin and corticotropin-releasing factor in the ovine fetal hypothalamus. Peptides. 1996;17:55–61. doi: 10.1016/0196-9781(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 22.Yu HK, Cabalum T, Jansen CA, Buster JE, Nathanielsz PW. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology. 1983;113:2216–2220. doi: 10.1210/endo-113-6-2216. [DOI] [PubMed] [Google Scholar]
- 23.Liggins GC, Schellenberg JC, Amato F, Godfrey B, Seamark RF. On the origin of dehydroepiandrosterone sulphate in the blood of fetal sheep. J. Endocrinol. 1985;104:279–283. doi: 10.1677/joe.0.1040279. [DOI] [PubMed] [Google Scholar]
- 24.Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol. Reprod. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- 25.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 26.Sklar CA, Mueller PL, Gluckman PD, Kaplan SL, Rudolph AM, Grumbach MM. Hormone ontogeny in the ovine fetus VII: circulating luteinizing hormone and follicle stimulating hormone in mid- and late- gestation. Endocrinology. 1981;108:874–880. doi: 10.1210/endo-108-3-874. [DOI] [PubMed] [Google Scholar]