Insulin resistance, obesity, and a sex hormone alteration have each been suggested as the underlying link for the constellation of risk factors for myocardial infarction (MI) commonly referred to as the "metabolic syndrome" or the "insulin-resistance syndrome." In an attempt to identify in women which of these variables is the most likely link, insulin, adiposity variables, sex hormones, and risk factors for MI were measured and their relationships analyzed statistically in 58 premenopausal and 20 postmenopausal healthy women. On controlling for age, visceral adipose tissue (VAT) correlated more strongly with risk factors for MI, insulin, and free testosterone (FT) than did total adipose tissue (TAT) or subcutaneous adipose tissue (SCAT). VAT, therefore, was used as the adiposity variable for further data analysis. Waist circumference (WC) was a better surrogate of VAT than was waist-to-hip ratio (WHR), which was a poor surrogate of VAT. VAT correlated positively with insulin, FT, triglyceride, and glucose, and negatively with HDL-C and sex-hormone binding globulin (SHBG). FT and insulin correlated with risk factors for MI, and with each other, on controlling for age, but on controlling for age and VAT, all of their correlations lost statistical significance except for FT-triglyceride and FT-insulin in the postmenopausal women. In conclusion, VAT accumulation in women, independently of other measures of adiposity, may largely explain the correlations of insulin, obesity, and sex hormones with risk factors for MI and may be the immediate underlying factor that links risk factors for MI to form the metabolic syndrome. Insulin resistance, which has been generally accepted to be the underlying factor, may be a component of the syndrome rather than its underlying link. We hypothesize that in women FT may effect preferential VAT accumulation and induce insulin resistance both directly and via VAT accumulation, so that a sex hormone alteration may underlie VAT accumulation and thus ultimately underlie the “metabolic syndrome” (with insulin resistance as a component).
The observation that glucose intolerance, hyperinsulinemia, hyperlipidemia, and hypertension concur to form a constellation of abnormalities that is found in various other clinical states as well as MI (1) supported the hypothesis that there is a factor underlying and linking these abnormalities (2). The identification of such an underlying factor could have profound implications for the prevention of atherosclerotic cardiovascular disease (CVD). The sex hormone milieu (1,2), insulin resistance (3.4), and obesity (1,5,6) have each been hypothesized as the factor underlying and linking these and other abnormalities to form the “metabolic” or “insulin resistance” syndrome. In a previous study evaluating these hypotheses in healthy men, we presented evidence suggesting that visceral adiposity is the immediate underlying link. We hypothesized that an alteration in the sex hormone milieu may underlie preferential VAT accumulation and also contribute to the insulin resistance in men (5). However, there appears to be an estrogen-androgen paradox in that in women, sex hormones are reported to relate oppositely from men to certain risk factors for MI, insulin, and coronary and aortic atherosclerosis (7). Our hypothesis for the development of the metabolic syndrome in men, therefore, may not be applicable to women.
In an attempt to determine and explain the relationships of insulin, adiposity variables, and sex hormones to risk factors for MI in women, the present study was carried out in 78 apparently healthy women on whom these variables, SHBG, and dehydroepiandrosterone sulfate (DHEAS) were measured. Because of the marked difference in estrogen status, the data on premenopausal and postmenopausal women were analyzed separately.
Patients and Methods
Seventy-eight adult females recruited from a multiethnic community through advertisements were studied. Inclusion required that the subject be healthy, not regularly participating in vigorous physical activity training programs (defined as >180 minutes of recreational physical activity per week, i.e., >30 minutes/day × six days/week), not having gained or lost >10% of body weight within the past year, not having a history of drug or alcohol abuse or cardiovascular disease, and not taking hormones or other medications known to influence serum lipid levels or body composition. The health of the subjects was determined by history, physical examination, and laboratory tests. Because blood pressure had not been measured according to standard procedure, blood pressure measurements are not included. Postmenopausal was defined as absence of menstruation for at least one year. The age range in the premenopausal women was 18 to 50 and in the postmenopausal women 46 to 81 years. This study was approved by the institutional review board and the subjects gave written informed consent.
BMI was calculated as weight/height (kg/m2). WC was measured at the narrowest point between the lowest rib and the iliac crest and hip circumference at the greatest protuberance of the buttocks.
TAT, SCAT, and VAT were measured using whole-body multislice magnetic resonance imaging as described in detail elsewhere (8). Subjects were in the supine position with arms extended over head. Using the inter-vertebral space between the fourth and fifth lumbar vertebrae as the origin, 10 mm thick transverse images were obtained every 40 mm from hand to foot. Image analysis software (Tomovision Inc., Montreal, Canada) was used to segment the cross-sectional images. The adipose tissue compartment was further segmented into subcutaneous and visceral components. Adipose tissue area (cm2) was calculated after segmentation by summing appropriate tissue pixels, then multiplying by individual pixel surface area. The volume of each tissue was calculated from slice areas and distances between slices using a mathematical algorithm.
Venous blood samples were drawn between 8 and 10 AM after a 12-hour overnight fast and the serum separated and stored at −20°C. Hormones were measured by radioimmunoassay (RIA). Materials for the RIA of estradiol (“3rd Generation” method), estrone, and SHBG were obtained from Diagnostic Systems Laboratories, Webster, TX, and for the RIA of total testosterone (TT), FT (nonprotein-bound testosterone), DHEAS, and insulin from Diagnostic Products, Los Angeles, CA. The interassay coefficient of variation for estradiol was 5.7% at 33.6 pg/ml, for estrone 6.9% at 36.7 pg/ml, for TT 9.5% at 0.74 ng/ml, for FT 19.3% at 1.35 pg/ml, for SHBG 4.8% at 104 nM/L, and for DHEAS 5.6% at 162.5 ug/dl. FT was also determined by calculation (cFT) (9) using the total testosterone and SHBG values (10). The correlation coefficient between FT and cFT in the 78 women was .84 (P<.001). Because blood samples were drawn at random times in the menstrual cycle, estrogen values in the premenopausal women were not analyzed. Serum cholesterol, triglyceride, and glucose were determined enzymatically, as was cholesterol in the supernatant following phosphotungstic acid precipitation of serum in the measurement of high-density lipoprotein cholesterol (HDL-C).
All statistical analyses were performed using SPSS version 10.0. Means ± SEM, ANOVA with age as covariate, and Pearson and partial correlations were calculated. A 2-tailed P value of < 0.05 was considered significant.
Results
Comparison of Variables between Premenopausal and Postmenopausal Women
Table 1 shows a comparison controlled for age of the means ± the standard errors of the means of the variables determined in the 58 premenopausal and 20 postmenopausal women. The adiposity variables were higher in the postmenopausal women and the adipose distribution markedly different from that of the premenopausal women. Although SCAT, which made up over 90% of TAT, was 37% higher in the postmenopausal women, VAT was approximately three times higher, so that VAT made up 4.3 % of TAT in the premenopausal women and 8.8% in the postmenopausal women. SCAT correlated with VAT in both the premenopausal (r=.71, P<.001) and postmenopausal (r=.67, P=.001) women. The mean values of the MI risk factors cholesterol, triglyceride, HDL-C, and glucose were higher in the postmenopausal women. The TT and FT levels were lower in the postmenopausal women. DHEAS was markedly lower in the postmenopausal women.
Table 1.
Comparison of the Means of Variables Controlled for Age between 58 Premenopausal and 20 Postmenopausal Women
| Variable | Mean ± SEM Premenopausal n = 58 | Postmenopausaln = 20 |
|---|---|---|
| Age, yr | 32.9 ± 1.2 | 61.4 ± 2.4 ‡ |
| Body weight, kg | 63.5 ± 1.6 | 69.3 ± 3.3 * |
| Body mass index, kg/m2 | 24.1 ± 0.6 | 27.9 ± 1.0 ‡ |
| Waist–to–hip ratio | 0.77 ± 0.01 | 0.85 ± 0.01 ‡ |
| Waist circumference, cm | 75.3 ± 1.2 | 86.6 ± 2.8 ‡ |
| Total adipose tissue, kg | 22.3 ± 1.3 | 32.0 ± 2.4 ‡ |
| Subcutaneous adipose tissue, kg | 21.3 ± 1.3 | 29.1 ± 2.2 ‡ |
| Visceral adipose tissue, kg | 0.96 ± 0.09 | 2.81 ± 0.30 ‡ |
| Cholesterol, mg/dl | 175 ± 4 | 229 ± 11 ‡ |
| Triglyceride, mg/dl | 77.3 ± 6.4 | 116 ± 16 † |
| HDL-cholesterol, mg/dl | 52.3 ± 1.6 | 59.8 ± 3.5 * |
| Cholesterol/HDL-cholesterol | 3.50 ± 0.12 | 4.00 ± 0.23 * |
| Glucose, mg/dl | 81.7 ± 1.1 | 89.9 ± 2.8 † |
| Insulin, µU/ml | 8.8 ± 0.6 | 9.6 ± 0.9 |
| Estradiol, pg/ml | 25.3 ± 2.7 | |
| Estrone, pg/ml | 28.0 ± 2.2 | |
| Testosterone, ng/ml | 0.32 ± 0.02 | 0.23 ± 0.03 ‡ |
| Free testosterone, pg/ml | 1.00 ± 0.08 | 0.70 ± 0.15* |
| Calculated free testosterone, pg/ml | 2.83 ± 0.20 | 2.58 ± 0.58 |
| DHEAS, µg/dl | 176 ± 13 | 73 ± 6 ‡ |
| Sex–hormone binding globulin, nmol/L | 146 ± 10 | 126 ± 14 |
P ≤ .05
P ≤ .01
P ≤ .001
Abbreviations: SEM, standard error of the mean; HDL- cholesterol, high–density lipoprotein-cholesterol; DHEAS, dehydroepiandrosterone sulfate.
Correlation Coefficients of Adiposity Variables and Age with Other Variables in the Premenopausal Women
Because WC correlated more strongly than WHR with VAT in both the premenopausal (r=.79 vs .48) and postmenopausal (r= .69 vs .46) women, it appeared to be a better surrogate of VAT than did WHR and the values for WHR are not shown in Table 2 and Table 3.
Table 2.
Correlation Coefficients of Adiposity Variables with Risk Factors for Myocardial Infarction and Hormones Controlled for Age in 58 Healthy Premenopausal Women
| Variable | TAT | SCAT | WC | VAT | Age |
|---|---|---|---|---|---|
| Age | .42§ | .40‡ | .55§ | .60§ | - |
| Cholesterol | .09 | .09 | .08 | .13 | .47§ |
| Triglyceride | .38‡ | .37‡ | .46§ | .50§ | −.19 |
| HDL-cholesterol | − .52§ | −.50§ | −.55§ | −.63§ | .22 |
| Glucose | .22 | .21 | .22 | .32 * | .12 |
| Insulin | .43§ | .42§ | .55§ | .43§ | .07 |
| Testosterone (T) | .22 | .22 | .11 | .11 | −.41§ |
| Free T | .30* | .29* | .32* | .40‡ | −.31* |
| Calculated free T | .35 † | .34 † | .33† | .41§ | −.44§ |
| SHBG | −.13 | −.11 | −.19 | −.38‡ | .02 |
| DHEAS | −.13 | −.13 | −.13 | −.04 | −.19 |
P ≤ .05
P ≤ .01
P ≤ .005
P≤.001.
Abbreviations: TAT, total adipose tissue; SCAT, subcutaneous adipose tissue; WC, waist circumference; VAT, visceral adipose tissue; HDL–cholesterol, high–density lipoprotein cholesterol; SHBG, sex-hormone binding globulin; DHEAS, dehydroepiandrosterone sulfate.
Table 3.
Correlation Coefficients of Adiposity Variables with Risk Factors for Myocardial Infarction and Hormones Controlled for Age in 20 Healthy Postmenopausal Women
| Variable | TAT | SCAT | WC | VAT | Age |
|---|---|---|---|---|---|
| Age | −.05 | −.05 | .08 | −.06 | - |
| Cholesterol | .04 | .04 | .06 | .08 | .21 |
| Triglyceride | .43 | .40 | .34 | .56† | .32 |
| HDL-cholesterol | −.25 | −.21 | −.26 | −.47* | −.08 |
| Glucose | −.03 | −.09 | .07 | .32 | .32 |
| Insulin | .37 | .32 | .51* | .58† | .13 |
| Estradiol | −.07 | −.08 | −.08 | −.00 | −.25 |
| Estrone | −.05 | −.04 | −.12 | −.09 | −.01 |
| Testosterone (T) | .43 | .41 | .59† | .42 | .02 |
| Free T | .42 | .39 | .55* | .51* | −.01 |
| Calculated freeT | .47 * | .44 | .60† | .53* | −.07 |
| SHBG | −.13 | −.08 | −.35 | −.39 | −.11 |
| DHEAS | −.26 | −.28 | −.04 | −.06 | −.34 |
P ≤ .05
P ≤ .01.
Abbreviations: TAT, total adipose tissue; SCAT, subcutaneous adipose tissue; WC, waist circumference; VAT, visceral adipose tissue; HDL–cholesterol, high–density lipoprotein cholesterol; SHBG, sex-hormone binding.
Table 2 shows the correlation coefficients of adiposity variables and age with risk factors for MI, insulin, sex hormones, SHBG, and DHEAS in the premenopausal women. The adiposity variables correlated positively and strongly with age, with the VAT-age correlation being the strongest. VAT correlated with age after controlling for TAT (r=.47, P<.001), SCAT (r=.49, P<.001), or WC (r=.32, P=.015). The splay of the VAT values as well appeared to increase with age (Fig. 1). The VAT/SCAT ratio also correlated positively with age in the premenopausal women alone (r=.47, P<.001) or combined with the postmenopausal women (r=0.65, P<0.001) (Fig. 2). Cholesterol correlated positively and TT, FT, and cFT negatively with age in the premenopausal women; on controlling for VAT, these correlations of cholesterol (r= 0.33, P=0.011), TT (r=−.39, P<.009), FT (r=−.48, P<.001), and cFT (r=−.44, P<.001) with age persisted. Because of these correlations with age, the correlations of the adiposity variables with risk factors for MI and hormones in Table 2 were controlled for age. VAT appeared to correlate more strongly than TAT and SCAT with triglyceride, HDL-C, and glucose, and similarly to TAT and SCAT with insulin. The Pearson correlation of VAT and cholesterol (r=.37, P=.004) became insignificant on controlling for age; none of the adiposity variables correlated with cholesterol on controlling for age. All of the correlations of TAT and SCAT with the risk factors and insulin became insignificant on controlling for age and VAT or VAT alone. On controlling for age, FT and cFT, but not TT, correlated with VAT and less strongly with TAT and SCAT. Controlling for age and VAT eliminated the correlations of FT and cFT with TAT and SCAT. SHBG correlated negatively with VAT on controlling for age or for age and insulin (r=−.37, P=.005) but did not correlate significantly with TAT or SCAT. On removing the two SHBG statistical outliers, this SHBG-VAT correlation became stronger and a correlation between SHBG and insulin controlled for age (r=−.33, P=.014) emerged but was eliminated on controlling for age and VAT. DHEAS did not correlate with any of the adiposity variables before or after controlling for age, but did correlate with TT (r=.50, P=<.001), FT (r=.55, P<.001), and cFT (r=.43, P=.001) on controlling for age and VAT. WHR was a poor surrogate of VAT, correlating only with age and insulin of the variables in Table 2.
Fig. 1.
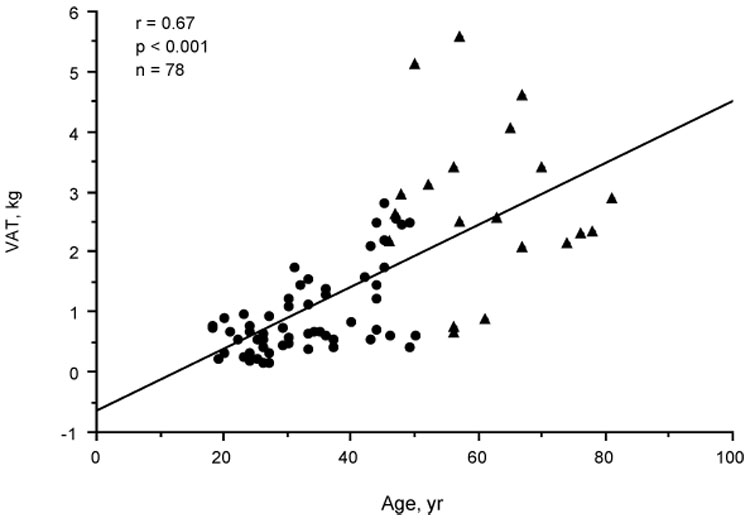
Scatterplot showing relationship of visceral adipose tissue level (VAT) to age in premenopausal (•) and postmenopausal (←) women.
Fig. 2.
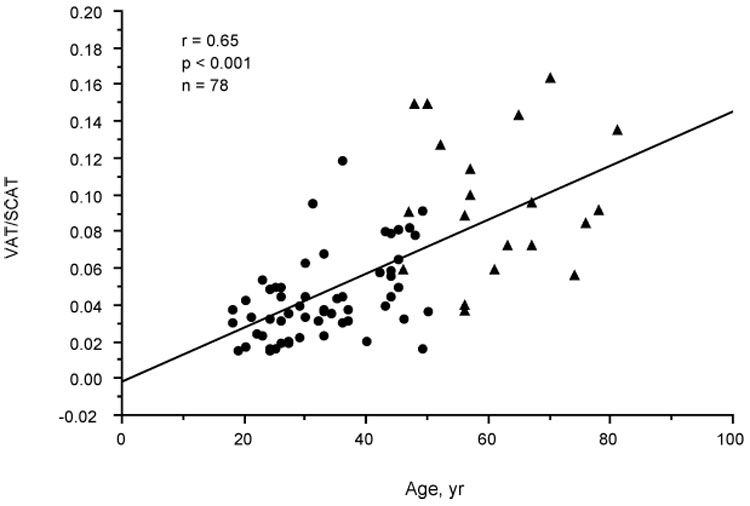
Scatterplot showing relationship of visceral adipose tissue/subcutaneous adipose tissue (VAT/SCAT) to age in premenopausal (•) and postmenopausal (←) women.
Correlation Coefficients of Adiposity Variables and Age with Other Variables in the Postmenopausal Women
Table 3 shows the corresponding data of Table 2 for the 20 postmenopausal women. None of the variables correlated significantly with age. However, VAT and VAT/SCAT appeared to follow the correlation line with age of the premenopausal women but with greater interindividual variation (Fig. 1 and Fig. 2). The VAT-controlled correlation of DHEAS with age approached significance (r=−.44, P=.06). As in the premenopausal women, VAT was a stronger correlate of triglyceride and HDL-C than were the other adiposity variables on controlling for age. Cholesterol showed no correlation with any of the adiposity variables. VAT, but not TAT or SCAT, correlated significantly with insulin. VAT correlated significantly with FT and cFT but not with TT. However, in contrast to the premenopausal women, the correlation of TT with VAT, TAT, and SCAT approached significance (P≤.08). The correlation coefficients of TT, FT, and cFT with VAT were the same before and after controlling for age. SHBG correlated with insulin on controlling for age (r=−.53, P=.020) but not on controlling for age and VAT. DHEAS did not correlate with the adiposity variables before or after controlling for age.
Although VAT correlated more strongly than WC with age, triglyceride, and HDL-C in the premenopausal women, WC correlated with these factors more strongly than TAT and SCAT and thus served as a better surrogate of VAT. WC correlated more strongly than VAT with insulin in the premenopausal women and with TT, FT, and cFT in the postmenopausal women. All of the significant correlations of WHR with the variables were stronger for WC. WHR was a poor surrogate for VAT.
Correlation Coefficients of Testosterone and Insulin with Risk Factors
Because VAT correlated more strongly with risk factors for MI, insulin, and FT than did TAT or SCAT, and controlling for VAT in addition to age eliminated the correlations of TAT and SCAT with these variables, VAT was used as the adiposity variable for further data analysis. Pearson correlations between insulin, sex hormones, and risk factors for MI in the premenopausal women were significant only for insulin with HDL-C (r=−.38, P=.003), insulin with triglyceride (r=.42, P=.001), FT with HDL-C (r=−.26, P=.049), and cFT with HDL-C (r=−.29, P=.027). Only the insulin-triglyceride (r=.42, P=.001) and insulin-HDL-C (r= −.41, P=.002) correlations remained significant after controlling for age and none were significant after controlling for age and VAT. Significant partial correlations among these variables in the postmenopausal women are shown in Table 4. On controlling for age, insulin correlated positively with glucose and approaching significance with triglyceride, FT and cFT correlated positively with triglyceride and negatively approaching significance with HDL-C, while TT, FT, and cFT correlated positively with insulin. On controlling for age and VAT, only the FT-triglyceride and FT-insulin correlations remained significant. SHBG correlated strongly with FT/TT on controlling for age and VAT in both the premenopausal (r=−.61, P<.001) and postmenopausal (r=−.66, P=.002) women.
Table 4.
Significant Partial Correlations of Sex Hormones, Insulin, and Risk Factors for Myocardial Infarction in 20 Healthy Postmenopausal Women
| Controlled for age | Controlled for age and VAT | |||
|---|---|---|---|---|
| r | P | r | P | |
| FT – triglyceride | .67 | .002 | .53 | .023 |
| Calculated FT – triglyceride | .58 | .009 | .40 | .097 |
| FT – HDL-cholesterol | −.45 | .056 | −.27 | .275 |
| Calculated FT – HDL-cholesterol | −.46 | .054 | −.29 | .251 |
| TT – insulin | .51 | .026 | .36 | .145 |
| FT – insulin | .69 | .001 | .56 | .015 |
| Calculated FT – insulin | .62 | .005 | .45 | .063 |
| Insulin – triglyceride | .45 | .053 | .18 | .470 |
| Insulin – glucose | .52 | .023 | .43 | .074 |
Abbreviations: FT, free testosterone; TT, total testosterone; HDL-cholesterol, high-density lipoprotein-cholesterol, VAT, visceral adipose tissue
Discussion
In a previous study in men, we found that VAT, which comprised only 11.5% of TAT, correlated more strongly than SCAT and TAT with risk factors for MI and that controlling for VAT eliminated the correlations of SCAT and TAT with risk factors for MI (5). In the present study, these relationships in both premenopausal and postmenopausal women were similar to those in men. The findings suggest that VAT, although it comprised only 4.3 and 8.8% of TAT in the premenopausal and postmenopausal women, respectively, may largely explain the correlations of obesity with risk factors found in women; VAT, therefore, was the adiposity variable used for further data analysis. Because all of the adiposity variables, and also VAT/SCAT, increased with age, correlations with adiposity variables were controlled for age.
On controlling for age and VAT, all of the significant correlations of insulin and FT with the risk factors were eliminated except for FT-triglyceride in the postmenopausal women. Thus, VAT accumulation may largely explain the correlations not only of obesity but also of insulin and testosterone with risk factors for MI in women, as previously found in men (5). The relationships of VAT with insulin, risk factors, and FT in this and other studies suggest that VAT accumulation could effect the expression of insulin resistance and risk factors for MI, as well as raise the FT level in women.
With regard to the relationship of VAT with glucose and insulin, in premenopausal women, the change in glucose area in the glucose tolerance test (11–13) and in insulin sensitivity (12,14) with weight change has been found to be more strongly related to change in VAT than in fat mass or SCAT. Removal of SCAT from obese diabetic or nondiabetic women by liposuction was reported to have no effect on insulin resistance or on the levels of glucose, insulin, or risk factors (15). That VAT may play a causal role in the expression of risk factors for MI in women is suggested by the decrease in risk factors with weight reduction (13,16), the preferential decrease in VAT with weight reduction (13,14,17,18), and the stronger association of VAT than SCAT with the risk factors. That weight reduction in obese premenopausal women caused a decrease in FT (19) suggests that VAT accumulation may cause a rise in FT. Thus, VAT accumulation could have a causal role in women, as well as in men (5), in the development of both insulin resistance and the risk factors for MI, and could also affect the testosterone level.
If VAT accumulation does cause the expression of insulin resistance and the risk factors and influence the testosterone level in women, these effects focus interest on factors other than weight gain that might influence VAT accumulation. Insulin resistance or sex hormone level, each of which is closely linked to obesity, are possible factors that could effect VAT accumulation as well as be affected by it.
With regard to insulin and VAT accumulation, insulin resistance or an increase in insulin level has been reported to predict a decrease rather than an increase in weight gain and a greater likelihood of weight loss in prospective studies in women as well as in men (20–23). A prospective study of a healthy nonobese population of both women and men with six-fold differences in insulin response to glucose showed that the baseline insulin response did not predict weight gain (24). Thus, it appears that insulin resistance or hyperinsulinemia, if anything, may mitigate VAT accumulation (20–23).
Although insulin resistance may not underlie, but may even mitigate, the VAT accumulation that appears to lead to the expression of risk factors, insulin resistance has been proposed to be (3,4) and is generally accepted to be the factor leading to the development of the metabolic or “insulin resistance” syndrome. In the present study, insulin-risk factor correlations were no longer significant when controlled for age and VAT. Thus, insulin resistance, which appears to be induced by VAT accumulation, may not be independently related to the risk factors and may be a component of the metabolic syndrome rather than the factor leading to it.
With regard to sex hormones, that testosterone may be a factor influencing VAT accumulation is suggested by the association of an increase in WC (25) and WHR (25,26) with hyperandrogenic states in women. In support is the observation that administration of testosterone to premenopausal women (27) and an androgen (28) to postmenopausal women preferentially increased VAT, whereas antiandrogen administration to premenopausal women with polycystic ovary syndrome preferentially decreased VAT (29). While neither estradiol nor estrone correlated with VAT in this study, the fact that the postmenopausal women had a VAT/SCAT ratio twice that of the premenopausal women without having a higher testosterone level raises the possibility that the sharp menopausal decline in estrogen level, rather than just an increase in weight, may have been a factor in this adipose redistribution. A significantly higher mean VAT/SCAT in postmenopausal women compared to premenopausal women of higher mean weight has been reported (30). The increase in VAT with age in the premenopausal women could have been owing to an attenuation of estrogen secretion with age (31,32). The development of an androgen excess relative to estrogen with menopause has been proposed (32) and may explain the increase in VAT/SCAT with age in women.
That the FT-insulin correlation in the postmenopausal women in this study remained significant on controlling for age and VAT suggests that FT may contribute directly to the insulin resistance in women, as well as indirectly by inducing accumulation of VAT. Administration of testosterone to healthy premenopausal (33) and postmenopausal (34) women has been reported to induce insulin resistance. Arguments have been presented previously that an elevation of the testosterone level may underlie insulin resistance in women (35). Thus, in women as well as in men (5), sex hormones may underlie the VAT accumulation that directly underlies the expression of risk factors for MI and insulin resistance, and may also lead directly to insulin resistance and possibly to certain risk factors for MI independently of VAT. Sex hormones, then, may ultimately underlie the metabolic syndrome (with hyperinsulinemia as a component of the syndrome) in women as previously hypothesized in men (1,5).
While testosterone related positively to insulin and risk factors for MI in women in the present study, testosterone related negatively to these factors in our previous study in men (5). In support of this apparent paradox, which has been noted previously (36,37), is the high free androgen index (38) and bioavailable testosterone level (39) in premenopausal women and free androgen index in postmenopausal women (35,40) reported to be associated with the metabolic syndrome, and the low TT level reported to be associated with (41,42) and prospective for (43) the metabolic syndrome in men. The positive relationship of testosterone to VAT found in this study in premenopausal and postmenopausal women and the negative relationship reported previously in men (44,45) suggest a similar paradox in the relationship of testosterone to VAT. Thus, if testosterone does underlie preferential VAT accumulation and VAT accumulation underlies the expression of insulin resistance and risk factors for MI in both women and men, the testosterone-VAT gender paradox is consistent with and may explain the similar gender paradox in the relationship of testosterone to insulin, risk factors for MI, and CVD (7).
VAT, but not TAT or SCAT, correlated significantly (inversely) with SHBG in the premenopausal women on controlling for age, suggesting that VAT may largely explain the correlation of obesity with SHBG in women. Data, not previously reported, from our study in men (5) similarly show that VAT correlated with SHBG (r=−.39, P<.001) more strongly than did TAT (r=−.31, P=.006) or SCAT (r=−.27, P=.015) on controlling for age.
The VAT-SHBG correlation also remained significant on controlling for age and insulin in the premenopausal women in the present study, whereas the insulin-SHBG correlation on controlling for age and VAT did not. Similarly, in the study in men (5), the VAT-SHBG correlation remained significant on controlling for age and insulin (r=−.28, P=.013), but insulin, which correlated significantly with SHBG on controlling for age and BMI (r=−.24, P=.033), as found previously (46), did not correlate significantly with SHBG on controlling for age and VAT. These observations suggest that VAT may correlate with SHBG independently of insulin and raise the possibility that VAT may be a more important determinant of SHBG level than is insulin.
In conclusion, the present study suggests that VAT (which appeared to increase with age at a faster rate than SCAT) may largely explain the constellation of abnormalities that occurs with adipose accumulation in both premenopausal and postmenopausal women. Insulin resistance, rather than being the factor leading to the metabolic syndrome in women, may instead be a component of it. An increased level of testosterone may effect the preferential accumulation of VAT in women. Thus, in women, as hypothesized previously in men, testosterone may underlie the VAT accumulation that appears to directly underlie the metabolic syndrome (with hyperinsulinemia as a component); testosterone may also contribute directly to insulin resistance and certain risk factors.
Interest has focused on determining the components of the metabolic syndrome. If the ultimate underlying factor were established, the components would be whatever abnormalities are associated with that factor (47). More importantly, the establishment of an underlying factor could present a more effective approach to the prevention of CVD.
Acknowledgments
Supported in part by National Institutes of Health Grant No. DK P01-42618
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips GB. Sex hormones, risk factors, and cardiovascular disease. Am J Med. 1978;65:7–11. doi: 10.1016/0002-9343(78)90685-x. [DOI] [PubMed] [Google Scholar]
- 2.Phillips GB. Relationship between serum sex hormones and glucose, insulin, and lipid abnormalities in men with myocardial infarction. Proc Natl Acad Sci USA. 1977;74:1729–1733. doi: 10.1073/pnas.74.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavaroni I, Dall’aglio E, Bonora E, Alpi O, Passeri M, Reaven GM. Evidence that multiple risk factors for coronary artery disease exist in persons with abnormal glucose tolerance. Am J Med. 1987;83:609–612. doi: 10.1016/0002-9343(87)90887-4. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Phillips GB, Jing T-Y, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 6.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 7.Phillips GB. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J Clin Endocrinol Metab. 2005;90:2708–2711. doi: 10.1210/jc.2004-2011. [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Wang Z, Tang H, Heshka S, Punyanitya M, Zhu S, Lei J, Heymsfield SB. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res. 2003;11:217–225. doi: 10.1038/oby.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol–17β to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 11.Despres J-P, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, Theriault G, Pinault S, Bouchard C. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux S, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Despres J-P. Seven-year changes in body fat and visceral adipose tissue in women. Diabetes Care. 1996;19:983–991. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- 13.Fujioka S, Matsuzawa Y, Tokunaga K, Seiichiro T. Improvement of glucose and lipid metabolism associated with selective reduction of intra- abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obesity. 1991;15:853–859. [PubMed] [Google Scholar]
- 14.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 15.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. New Eng J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 16.Olefsky J, Reaven GM, Farquhar JW. Effects of weight reduction on obesity. Studies on lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest. 1974;53:64–76. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamboni M, Armellini F, Turcato E, Todesco T, Bissoli L, Bergamo-Andreis A, et al. Effect of weight loss on body fat distribution in premenopausal women. Am J Clin Nutr. 1993;58:29–34. doi: 10.1093/ajcn/58.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Smith SR, Zachwieja JJ. Visceral adipose tissue: a critical review of intervention strategies. Int J Obesity. 1999;23:329–335. doi: 10.1038/sj.ijo.0800834. [DOI] [PubMed] [Google Scholar]
- 19.Kopelman PG, White N, Pilkington TR, Jeffcoate SL. The effect of weight loss on sex steroid secretion and binding in massively obese women. Clin Endocrinol (Oxf) 1981;15:113–116. doi: 10.1111/j.1365-2265.1981.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 20.Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdez R, Mitchell BD, Haffner SM, Hazuda HP, Morales PA, Monterrosa A, et al. Predictors of weight change in a bi-ethnic population. The San Antonio Heart Study. Int J Obes Relat Metab Disord. 1994;18:85–91. [PubMed] [Google Scholar]
- 22.Hoag S, Marshall JA, Jones RH, Hamman RF. High fasting insulin levels associated with lower rates of weight gain in persons with normal glucose tolerance: the San Luis Valley Diabetes Study. Int J Obes Relat Metab Disord. 1995;19:175–180. [PubMed] [Google Scholar]
- 23.Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. J Clin Endocrinol Metab. 1995;80:1571–1576. doi: 10.1210/jcem.80.5.7745002. [DOI] [PubMed] [Google Scholar]
- 24.Zavaroni I, Zuccarelli A, Gasparini P, Massironi P, Barilli A, Reaven GM. Can weight gain in healthy, nonobese volunteers be predicted by differences in baseline plasma insulin concentration? J Clin Endocrinol Metab. 1998;83:3498–3500. doi: 10.1210/jcem.83.10.5178. [DOI] [PubMed] [Google Scholar]
- 25.Hauner H, Ditschuneit HH, Pal SB, Moncayo R, Pfeiffer EF. Fat distribution, endocrine and metabolic profile in obese women with and without hirsutism. Metabolism. 1988;37:281–286. doi: 10.1016/0026-0495(88)90109-6. [DOI] [PubMed] [Google Scholar]
- 26.Evans DJ, Barth JH, Burke CW. Body fat topography in women with androgen excess. Int J Obesity. 1988;12:157–162. [PubMed] [Google Scholar]
- 27.Elbers JMH, Asscheman H, Seidell JC, Megens JAJ, Gooren LJG. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82:2044–2047. doi: 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy JC, Bray GA, Bourgeois MO, Macchiavelli R, Rood JC, Greeson C, et al. Exogenous androgens influence body composition and regiona body fat distribution in obese postmenopausal women--a clinical research center study. J Clin Endocrinol Metab. 1996;81:2198–2203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 29.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol. 2004;60:241–249. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 30.Zamboni M, Armellini F, Milani MP, De Marchi M, Todesco T, Robbi R, et al. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obesity. 1992;16:495–504. [PubMed] [Google Scholar]
- 31.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Ding J, Bush TL, Longenecker JC, Nieto FJ, Golden SH, et al. Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidem. 1999;154:489–494. doi: 10.1093/aje/154.6.489. [DOI] [PubMed] [Google Scholar]
- 33.Polderman KH, Gooren LJG, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–271. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- 34.Zang H, Carlstrom K, Arner P, Hirscherg AL. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril. 2006;86:136–144. doi: 10.1016/j.fertnstert.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women. The Atherosclerosis Risk in Communities Study. Am J Epidem. 2004;160:540–548. doi: 10.1093/aje/kwh250. [DOI] [PubMed] [Google Scholar]
- 36.Phillips GB. Relationship of serum sex hormones to coronary heart disease. Steroids. 1993;58:286–290. 554–555. doi: 10.1016/0039-128x(93)90075-x. [DOI] [PubMed] [Google Scholar]
- 37.Phillips GB, Pinkernell BH, Jing T-Y. Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:695–701. doi: 10.1161/01.atv.17.4.695. [DOI] [PubMed] [Google Scholar]
- 38.Korhonen S, Hippelainen M, Vanhala M, Heinonen S, Niskanen L. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril. 2003;79:1327–1334. doi: 10.1016/s0015-0282(03)00347-9. [DOI] [PubMed] [Google Scholar]
- 39.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg ML, Manson JE, Buring JE, Cook NR, Seely EW, Ridker PM, et al. Low sex hormone-binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism. 2006;55:1473–1480. doi: 10.1016/j.metabol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T, Salonen R. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Europ J Endocrinol. 2003;149:601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 42.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncology. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 43.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T, Valkonen V, et al. Testosterone and sex hormone binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 44.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes. 2000;24:485–491. doi: 10.1038/sj.ijo.0801183. [DOI] [PubMed] [Google Scholar]
- 45.Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes. 1992;16:991–997. [PubMed] [Google Scholar]
- 46.Phillips GB. Relationship between serum sex hormones and the glucose-insulin-lipid defect in men with obesity. Metabolism. 1993;42:116–120. doi: 10.1016/0026-0495(93)90181-m. [DOI] [PubMed] [Google Scholar]
- 47.Phillips GB. The GIHLT-E syndrome. Diabetes Care. 2004;27:2285–2286. doi: 10.2337/diacare.27.9.2285-a. [DOI] [PubMed] [Google Scholar]


