Abstract
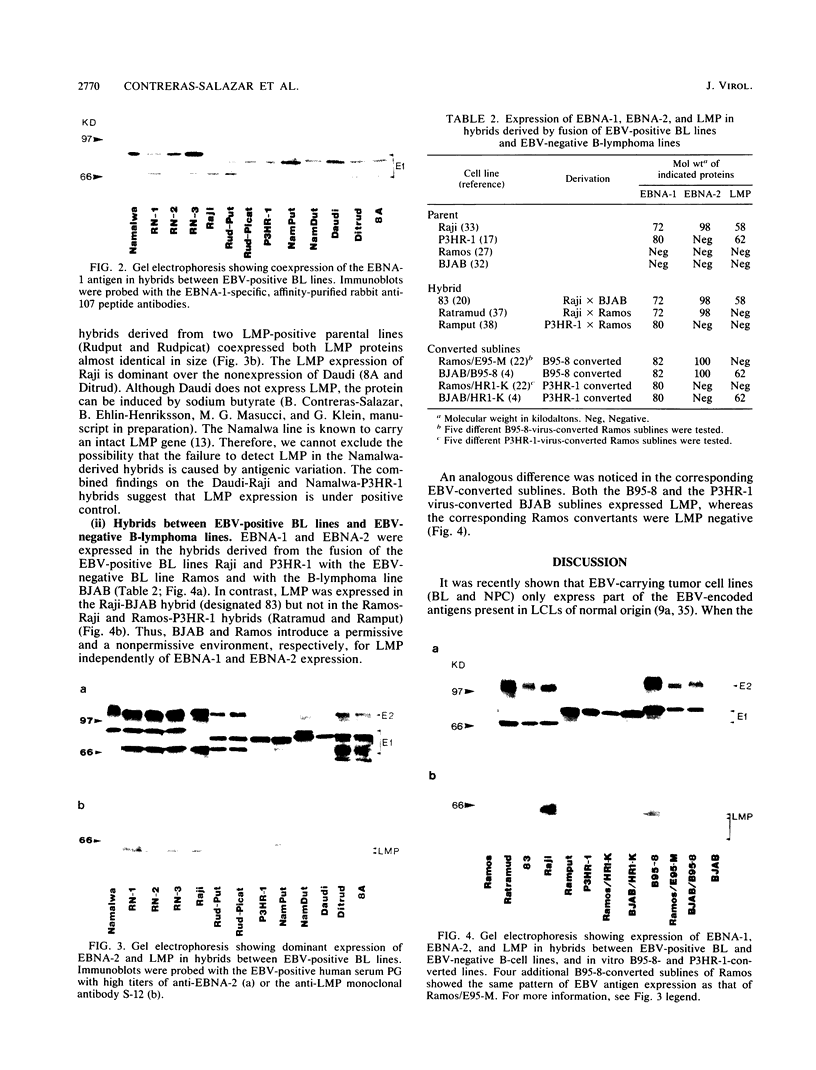
We have analyzed the expression of the three major known growth transformation-associated Epstein-Barr virus (EBV) proteins, EBNA-1, EBNA-2, and latent membrane protein (LMP), in a series of somatic cell hybrids derived from the fusion of EBV-carrying Burkitt lymphoma (BL) lines with EBV-positive or EBV-negative B-cell lines. Independently of the cell phenotype, EBNA-1 was invariably coexpressed in all EBV-carrying hybrids. In hybrids between EBV-carrying, LMP-positive and LMP-negative Burkitt lymphoma lines, LMP was expressed, indicating positive control. Two EBV-negative lymphoma lines, Ramos and BJAB, differed in their ability to express LMP after B95-8 virus-induced conversion and after hybridization with Raji cells. BJAB was permissive while Ramos was nonpermissive for LMP, although both expressed EBNA-2. The EBNA-2-deleted P3HR-1 virus gave the same pattern of LMP expression in these two cells. Our findings indicate that the expression of EBNA-1, EBNA-2, and LMP is regulated by independent mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Clements G. B., Klein G., Povey S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int J Cancer. 1975 Jul 15;16(1):125–133. doi: 10.1002/ijc.2910160114. [DOI] [PubMed] [Google Scholar]
- Clements G. B. Selection of biochemically variant clones of human lymphoid cells. Ann Hum Genet. 1976 Jan;39(3):353–360. doi: 10.1111/j.1469-1809.1976.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B., Alexander H., Ernberg I., Uno M., Ono Y., Klein G., Lerner R. A. An Epstein-Barr virus (EBV)-determined nuclear antigen (EBNA5) partly encoded by the transformation-associated Bam WYH region of EBV DNA: preferential expression in lymphoblastoid cell lines. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6641–6645. doi: 10.1073/pnas.83.17.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B., Ehlin-Henriksson B., Rymo L., Henle W., Henle G., Klein G. The Epstein-Barr virus determined nuclear antigen is composed of at least three different antigens. Int J Cancer. 1986 Feb 15;37(2):195–200. doi: 10.1002/ijc.2910370205. [DOI] [PubMed] [Google Scholar]
- Dillner J., Sternås L., Kallin B., Alexander H., Ehlin-Henriksson B., Jörnvall H., Klein G., Lerner R. Antibodies against a synthetic peptide identify the Epstein-Barr virus-determined nuclear antigen. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4652–4656. doi: 10.1073/pnas.81.15.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Gergely L., Sternås L., Dillner J., Klein G. Molecular size variation of EBNA is determined by the EB viral genome. Intervirology. 1984;22(2):85–96. doi: 10.1159/000149538. [DOI] [PubMed] [Google Scholar]
- Greenberg A., Ber R., Kra-Oz Z., Laskov R. Extinction of expression of immunoglobulin genes in myeloma X fibroblast somatic cell hybrids. Mol Cell Biol. 1987 Feb;7(2):936–939. doi: 10.1128/mcb.7.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A., Ripley S., Heller M., Kieff E. Chromosome site for Epstein-Barr virus DNA in a Burkitt tumor cell line and in lymphocytes growth-transformed in vitro. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1987–1991. doi: 10.1073/pnas.80.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Jones M. D., Foster L., Sheedy T., Griffin B. E. The EB virus genome in Daudi Burkitt's lymphoma cells has a deletion similar to that observed in a non-transforming strain (P3HR-1) of the virus. EMBO J. 1984 Apr;3(4):813–821. doi: 10.1002/j.1460-2075.1984.tb01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Dillner J., Ernberg I., Ehlin-Henriksson B., Rosén A., Henle W., Henle G., Klein G. Four virally determined nuclear antigens are expressed in Epstein-Barr virus-transformed cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1499–1503. doi: 10.1073/pnas.83.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Klein G., Clements G., Zeuthen J., Westman A. Somatic cell hybrids between human lymphoma lines. II. Spontaneous and induced patterns of the Epstein-Barr virus (EBV) cycle. Int J Cancer. 1976 Jun 15;17(6):715–724. doi: 10.1002/ijc.2910170605. [DOI] [PubMed] [Google Scholar]
- Klein G., Ehlin-Henriksson B., Schlossman S. F. Induction of an activated b lymphocyte-associated surface moiety defined by the B2 monoclonal antibody by ebv conversion of an EBV-negative lymphoma line (Ramos): differential effect of transforming (B95-8) and nontransforming (P3HR-1) EBV substrains. J Immunol. 1983 Apr;130(4):1985–1989. [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Eriksson I., Terasaki P., Bernoco M., Rosén A., Masucci G., Povey S., Ber R. Hybridization of a myeloid leukemia-derived human cell line (K562) with a human Burkitt's lymphoma line (P3HR-1). J Natl Cancer Inst. 1980 Apr;64(4):725–738. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci M. G., Torsteindottir S., Colombani J., Brautbar C., Klein E., Klein G. Down-regulation of class I HLA antigens and of the Epstein-Barr virus-encoded latent membrane protein in Burkitt lymphoma lines. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4567–4571. doi: 10.1073/pnas.84.13.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Misko I. S., Burrows S. R., Burman K., McCarthy R., Sculley T. B. Cytotoxic T-cell clones discriminate between A- and B-type Epstein-Barr virus transformants. Nature. 1988 Feb 25;331(6158):719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Wang D., Young L. S., Wang F., Rowe M., Kieff E., Rickinson A. B. Epstein-Barr virus-specific cytotoxic T-cell recognition of transfectants expressing the virus-coded latent membrane protein LMP. J Virol. 1988 Oct;62(10):3747–3755. doi: 10.1128/jvi.62.10.3747-3755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. J., Young L. S., Calender A., Gregory C. D., Rowe M., Lenoir G. M., Rickinson A. B. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J Virol. 1988 Mar;62(3):894–901. doi: 10.1128/jvi.62.3.894-901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., DeJesus E., Dugan D., Croce C. M. Repression of rearranged mu gene and translocated c-myc in mouse 3T3 cells X Burkitt lymphoma cell hybrids. Science. 1984 Apr 27;224(4647):399–402. doi: 10.1126/science.6424234. [DOI] [PubMed] [Google Scholar]
- Nyormoi O., Klein G., Adams A., Dombos L. Sensitivity to EBV superinfection and IUdR inducibility of hybrid cells formed between a sensitive and a relatively resistant Burkitt lymphoma cell line. Int J Cancer. 1973 Sep 15;12(2):396–408. doi: 10.1002/ijc.2910120211. [DOI] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Ricksten A., Kallin B., Alexander H., Dillner J., Fåhraeus R., Klein G., Lerner R., Rymo L. BamHI E region of the Epstein-Barr virus genome encodes three transformation-associated nuclear proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(4):995–999. doi: 10.1073/pnas.85.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Rowe M., Evan G. I., Wallace L. E., Farrell P. J., Rickinson A. B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986 Oct;5(10):2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira G., Aman P., Silvian I., Koide N., Ber R., Klein G. Membrane-bound and cellular immunoglobulins in human B-lymphoma lines and derived hybrids. Immunobiology. 1981;160(3-4):340–351. doi: 10.1016/s0171-2985(81)80060-5. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Israelsohn E. S. Generation of specific cytotoxic T cells with a fragment of the Epstein-Barr virus-encoded p63/latent membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5384–5388. doi: 10.1073/pnas.84.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsteinsdóttir S., Andersson M. L., Avila-Cariño J., Ehlin-Henriksson B., Masucci M. G., Klein G., Klein E. Reversion of tumorigenicity and decreased agarose clonability after EBV conversion of an IgH/myc translocation-carrying BL line. Int J Cancer. 1989 Feb 15;43(2):273–278. doi: 10.1002/ijc.2910430219. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Zeuthen J., Klein G., Ber R., Masucci G., Bisballe S., Povey S., Terasaki P., Ralph P. Human lymphoma-lymphoma hybrids and lymphoma-leukemia hybrids. I. Isolation, characterization, cell surface markers, and B-cell markers. J Natl Cancer Inst. 1982 Feb;68(2):179–195. [PubMed] [Google Scholar]