Abstract
Exposure to the tiglian 12-O-tetradecanoylphorbol-13-acetate (TPA) represents one of the most efficient and widely used protocols for inducing Epstein-Barr virus (EBV)-infected cells from latent into lytic cycle. Since TPA is both a potent tumor promoter and a potent activator of the cellular protein kinase C (PKC), we sought to determine whether either of these activities was closely linked to EBV lytic cycle induction. A panel of TPA structural analogs, encompassing tiglians with different spectra of biological activities, was assayed on a number of EBV-positive B-lymphoid cell lines. Lytic cycle induction correlated with the capacity to activate PKC, not with tumor promoter status; some nonpromoting tiglians were as efficient as TPA in inducing lytic cycle antigen expression. We then sought more direct evidence for an involvement of PKC in the induction process. In initial experiments, 1-(5-isoquinolinyl sulphonyl)-2-methylpiperazine (H-7), the best available pharmacological inhibitor of PKC, completely blocked the induction of the lytic cycle by TPA and its active analogs. This is consistent with, but does not prove, a requirement for active PKC in the induction process, since H-7 targets PKC preferentially but also has some effects on other kinases. We therefore turned to the synthetic pseudosubstrate peptide PKC(19-36) as a means of specific PKC inhibition and to the closely related but inactive peptide PKC(19-Ser-25-36) as a control. Using the technique of scrape loading to deliver the peptides into cells of an adherent EBV-positive target line, we found that the pseudosubstrate peptide PKC(19-36) completely and specifically blocked tiglian-induced entry of the cells into the lytic cycle. The evidence both from TPA analogs and from enzyme inhibition studies therefore indicates that the pathway linking TPA treatment to lytic cycle induction involves active PKC. Interestingly, inhibition of PKC had no effect upon the spontaneous entry into lytic cycle which occurs in naturally productive cell lines, suggesting that spontaneous entry is signalled by another route.
Full text
PDF

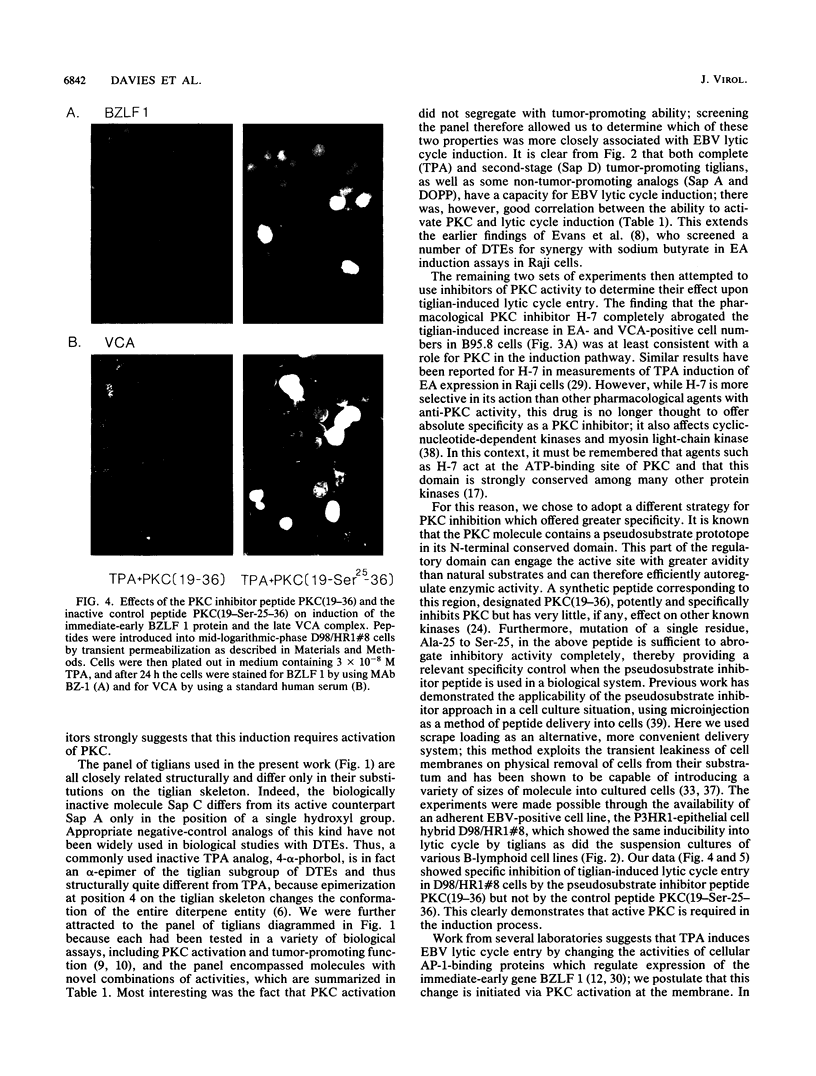


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks G., Evans A. T., Aitken A., Evans F. J. Tumour-promoting and hyperplastic effects of phorbol and daphnane esters in CD-1 mouse skin and a synergistic effect of calcium ionophore with the non-promoting activator of protein kinase C, sapintoxin A. Carcinogenesis. 1989 Feb;10(2):283–288. doi: 10.1093/carcin/10.2.283. [DOI] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. H., Ando I. EB virus induction is associated with B-cell maturation. Immunology. 1986 Nov;59(3):405–409. [PMC free article] [PubMed] [Google Scholar]
- Daibata M., Humphreys R. E., Takada K., Sairenji T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J Immunol. 1990 Jun 15;144(12):4788–4793. [PubMed] [Google Scholar]
- Edwards M. C., Taylor S. E., Williamson E. M., Evans F. J. New phorbol and deoxyphorbol esters: isolation and relative potencies in inducing platelet aggregation and erythema of skin. Acta Pharmacol Toxicol (Copenh) 1983 Sep;53(3):177–187. doi: 10.1111/j.1600-0773.1983.tb01122.x. [DOI] [PubMed] [Google Scholar]
- Evans A. T., Brooks G., Evans F. J. Non-promoting diterpene esters can induce Epstein-Barr virus early antigen expression in the Raji cell line. Cancer Lett. 1990 Jan;49(1):25–29. doi: 10.1016/0304-3835(90)90135-k. [DOI] [PubMed] [Google Scholar]
- Evans F. J., Parker P. J., Olivier A. R., Thomas S., Ryves W. J., Evans A. T., Gordge P., Sharma P. Phorbol ester activation of the isotypes of protein kinase C from bovine and rat brain. Biochem Soc Trans. 1991 Apr;19(2):397–402. doi: 10.1042/bst0190397. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Rowe D. T., Rooney C. M., Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989 Jan;8(1):127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., O'Neill F. J. Hybridization of Burkitt lymphoblastoid cells. Science. 1972 Jun 16;176(4040):1245–1247. doi: 10.1126/science.176.4040.1245. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Rowe M., Rickinson A. B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990 Jul;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hardie G. Pseudosubstrates turn off protein kinases. Nature. 1988 Oct 13;335(6191):592–593. doi: 10.1038/335592a0. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Kenney S., Holley-Guthrie E., Mar E. C., Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989 Sep;63(9):3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishishita M., Luka J., Vroman B., Poduslo J. F., Pearson G. R. Production of monoclonal antibody to a late intracellular Epstein-Barr virus-induced antigen. Virology. 1984 Mar;133(2):363–375. doi: 10.1016/0042-6822(84)90402-1. [DOI] [PubMed] [Google Scholar]
- Lazdins J., Zompetta C., Grimaldi S., Barile G., Venanzoni M., Frati L., Faggioni A. TPA induction of Epstein-Barr virus early antigens in Raji cells is blocked by selective protein kinase-C inhibitors. Int J Cancer. 1987 Dec 15;40(6):846–849. doi: 10.1002/ijc.2910400624. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Sample J., Hayward G. S., Hayward S. D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990 Mar;64(3):1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington S. R., Hesketh T. R., Metcalfe J. C. GTP gamma S activation of proto-oncogene expression in transiently permeabilised Swiss 3T3 fibroblasts. FEBS Lett. 1988 Jan 25;227(2):203–208. doi: 10.1016/0014-5793(88)80899-8. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Buck W. R. A synthetic peptide of the pseudosubstrate domain of protein kinase C blocks cytoplasmic alkalinization during activation of the sea urchin egg. Dev Biol. 1990 Aug;140(2):272–280. doi: 10.1016/0012-1606(90)90077-v. [DOI] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Oguro M., Ono Y. Identification of coding regions for various Epstein-Barr virus-specific antigens by gene transfer and serology. J Virol. 1986 Oct;60(1):324–330. doi: 10.1128/jvi.60.1.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Lenoir G., Begon-Lours J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978 Nov 16;276(5685):270–272. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- Yao Q. Y., Ogan P., Rowe M., Wood M., Rickinson A. B. Epstein-Barr virus-infected B cells persist in the circulation of acyclovir-treated virus carriers. Int J Cancer. 1989 Jan 15;43(1):67–71. doi: 10.1002/ijc.2910430115. [DOI] [PubMed] [Google Scholar]
- Young L. S., Lau R., Rowe M., Niedobitek G., Packham G., Shanahan F., Rowe D. T., Greenspan D., Greenspan J. S., Rickinson A. B. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991 Jun;65(6):2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]