Abstract
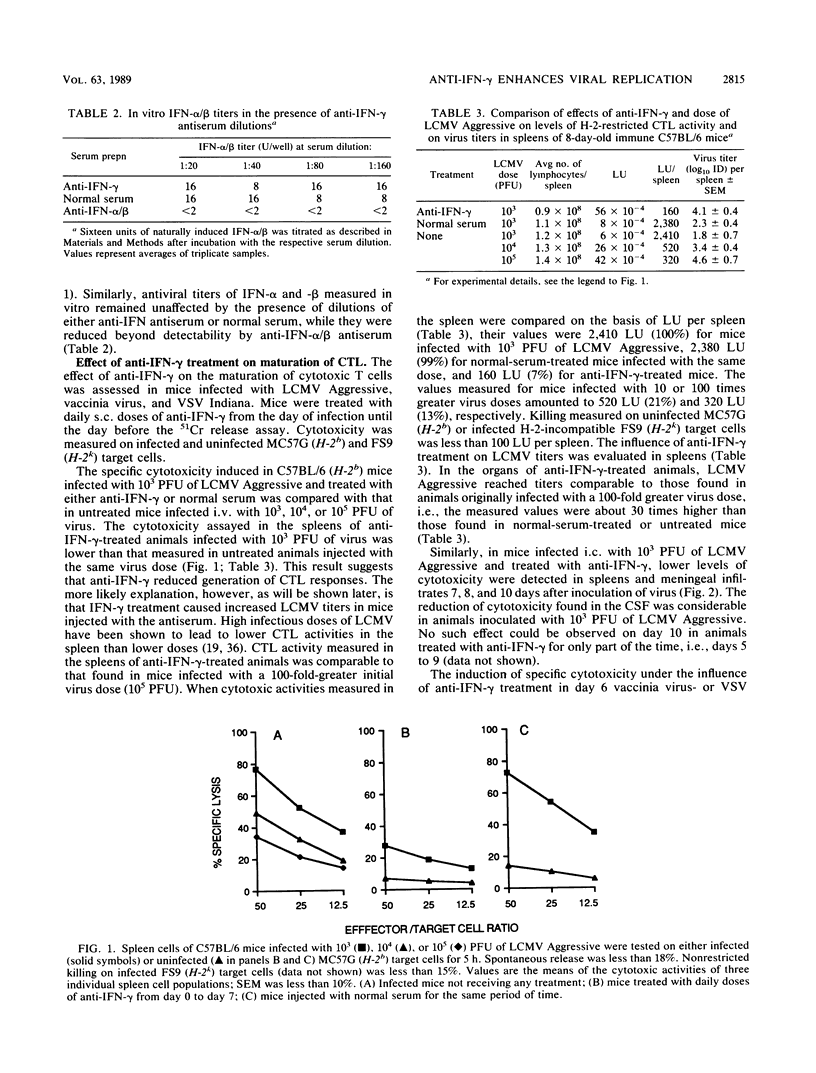
The role of gamma interferon (IFN-gamma) induced during a viral infection in the ability of the host to acquire antiviral immunity was studied in mice. They were injected subcutaneously daily with an ammonium sulfate-precipitated sheep anti-IFN-gamma antibody preparation able to neutralize 10(4) U of IFN-gamma. Specificity of the anti-IFN-gamma antiserum was demonstrated by absence of detectable activity against natural IFN-alpha and -beta. Controls were treated with a similarly prepared normal sheep serum. Treatment with the IFN-gamma-specific antibody preparation had no influence on the ability of mice to generate anti-vaccinia virus- or anti-vesicular stomatitis virus (VSV)-specific cytotoxic T-cell (CTL) responses or T helper-dependent immunoglobulin G responses to VSV. In contrast, treatment of mice with sheep anti-IFN-gamma impaired CTL responses against lymphocytic choriomeningitis (LCM) virus (LCMV, Aggressive isolate); in addition, under the experimental conditions used, it prevented lethal LCM. Cytotoxic T-cell activity measured in the spleens of anti-IFN-gamma-treated mice was comparable to that found in mice initially infected with a 100-fold-larger dose of LCMV. Evaluation of the effects of treatment on the kinetics of virus replication revealed that in both euthymic and athymic nude C57BL/6 mice, anti-IFN-gamma treatment led to an increase of virus titers up to 100-fold compared with control mice. Therefore, IFN-gamma may play a role in controlling viruses with tropism for lymphocytes and monocytes/macrophages, such as LCMV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Oldstone M. B. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988 May 1;167(5):1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Davidson A. L., Merz P. A. A method for obtaining cerebrospinal fluid from mice. Res Vet Sci. 1971 Sep;12(5):499–499. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Charan S., Zinkernagel R. M. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J Immunol. 1986 Apr 15;136(8):3057–3061. [PubMed] [Google Scholar]
- De Maeyer-Guignard J., De Maeyer E. Immunomodulation by interferons: recent developments. Interferon. 1985;6:69–91. [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Frei K., Leist T. P., Meager A., Gallo P., Leppert D., Zinkernagel R. M., Fontana A. Production of B cell stimulatory factor-2 and interferon gamma in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J Exp Med. 1988 Jul 1;168(1):449–453. doi: 10.1084/jem.168.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Coppey J., Falcoff E., Fontaine D. Interferon and murine leukemia. I. Inhibitory effect of interferon preparations on development of friend leukemia in mice. Proc Soc Exp Biol Med. 1967 Jan;124(1):84–91. doi: 10.3181/00379727-124-31672. [DOI] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Jacobson S., Pfau C. J. Viral pathogenesis and resistance to defective interfering particles. Nature. 1980 Jan 17;283(5744):311–313. doi: 10.1038/283311a0. [DOI] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Aguet M., Hässig M., Pevear D. C., Pfau C. J., Zinkernagel R. M. Lack of correlation between serum titres of interferon alpha, beta, natural killer cell activity and clinical susceptibility in mice infected with two isolates of lymphocytic choriomeningitis virus. J Gen Virol. 1987 Aug;68(Pt 8):2213–2218. doi: 10.1099/0022-1317-68-8-2213. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968 Oct;101(4):717–724. [PubMed] [Google Scholar]
- Pfau C. J., Valenti J. K., Pevear D. C., Hunt K. D. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J Exp Med. 1982 Jul 1;156(1):79–89. doi: 10.1084/jem.156.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig S., Laskay T., Andersson J., De Ley M., Andersson U. Gamma-interferon is produced by CD3+ and CD3- lymphocytes. Immunol Rev. 1987 Jun;97:51–65. doi: 10.1111/j.1600-065x.1987.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheynius A., Johansson C., van der Meide P. H. In vivo induction of Ia antigens on rat keratinocytes by gamma-interferon. Br J Dermatol. 1986 Nov;115(5):543–549. doi: 10.1111/j.1365-2133.1986.tb05763.x. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Talmadge J. E., Herberman R. B., Chirigos M. A., Maluish A. E., Schneider M. A., Adams J. S., Philips H., Thurman G. B., Varesio L., Long C. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J Immunol. 1985 Oct;135(4):2483–2491. [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Wentworth P. A., Ziegler H. K. Induction of macrophage Ia expression by lipopolysaccharide and Listeria monocytogenes in congenitally athymic nude mice. J Immunol. 1987 May 15;138(10):3167–3173. [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R. One-signal requirement for interferon-gamma production by human large granular lymphocytes. J Immunol. 1987 Aug 1;139(3):724–727. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Cytotoxic thymus-derived lymphocytes in cerebrospinal fluid of mice with lymphocytic choriomeningitis. J Exp Med. 1973 Nov 1;138(5):1266–1269. doi: 10.1084/jem.138.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Leist T., Hengartner H., Althage A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J Exp Med. 1985 Dec 1;162(6):2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]



