Abstract
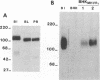
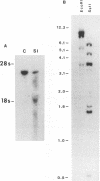
The cellular receptor for murine coronavirus mouse hepatitis virus (MHV)-A59 is a member of the carcinoembryonic antigen (CEA) family of glycoproteins in the immunoglobulin superfamily. We isolated a cDNA clone (MHVR1) encoding the MHV receptor. The sequence of this clone predicts a 424-amino-acid glycoprotein with four immunoglobulinlike domains, a transmembrane domain, and a short intracytoplasmic tail, MHVR1 is closely related to the murine CEA-related clone mmCGM1 (Mus musculus carcinoembryonic antigen gene family member). Western blot (immunoblot) analysis performed with antireceptor antibodies detected a glycoprotein of 120 kDa in BHK cells stably transfected with MHVR1. This corresponds to the size of the MHV receptor expressed in mouse intestine and liver. Human and hamster fibroblasts transfected with MHVR1 became susceptible to infection with MHV-A59. Like MHV-susceptible mouse fibroblasts, the MHVR1-transfected human and hamster cells were protected from MHV infection by pretreatment with monoclonal antireceptor antibody CC1. Thus, the 110- to 120-kDa CEA-related glycoprotein encoded by MHVR1 is a functional receptor for murine coronavirus MHV-A59.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Benchimol S., Cournoyer D., Fuks A., Stanners C. P. Isolation and characterization of full-length functional cDNA clones for human carcinoembryonic antigen. Mol Cell Biol. 1987 Sep;7(9):3221–3230. doi: 10.1128/mcb.7.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Turbide C., Afar D., Bell J., Raymond M., Stanners C. P., Fuks A. A mouse analogue of the human carcinoembryonic antigen. Cancer Res. 1989 Apr 15;49(8):2017–2021. [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Brian D. A., Enjuanes L., Holmes K. V., Lai M. M., Laude H., Siddell S. G., Spaan W., Taguchi F., Talbot P. J. Recommendations of the Coronavirus Study Group for the nomenclature of the structural proteins, mRNAs, and genes of coronaviruses. Virology. 1990 May;176(1):306–307. doi: 10.1016/0042-6822(90)90259-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Frana M. F., Behnke J. N., Sturman L. S., Holmes K. V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985 Dec;56(3):912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., von Bonsdorff C. H., Simons K. Cell surface influenza haemagglutinin can mediate infection by other animal viruses. EMBO J. 1985 Oct;4(10):2475–2485. doi: 10.1002/j.1460-2075.1985.tb03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoda Y., Neumaier M., Hefta S. A., Drzeniek Z., Wagener C., Shively L., Hefta L. J., Shively J. E., Paxton R. J. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6959–6963. doi: 10.1073/pnas.85.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Goto N., Ogawa T., Ono K., Murakami T., Fujiwara K. Hydrocephalus in suckling rats infected intracerebrally with mouse hepatitis virus, MHV-A59. Microbiol Immunol. 1980;24(9):825–834. doi: 10.1111/j.1348-0421.1980.tb02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
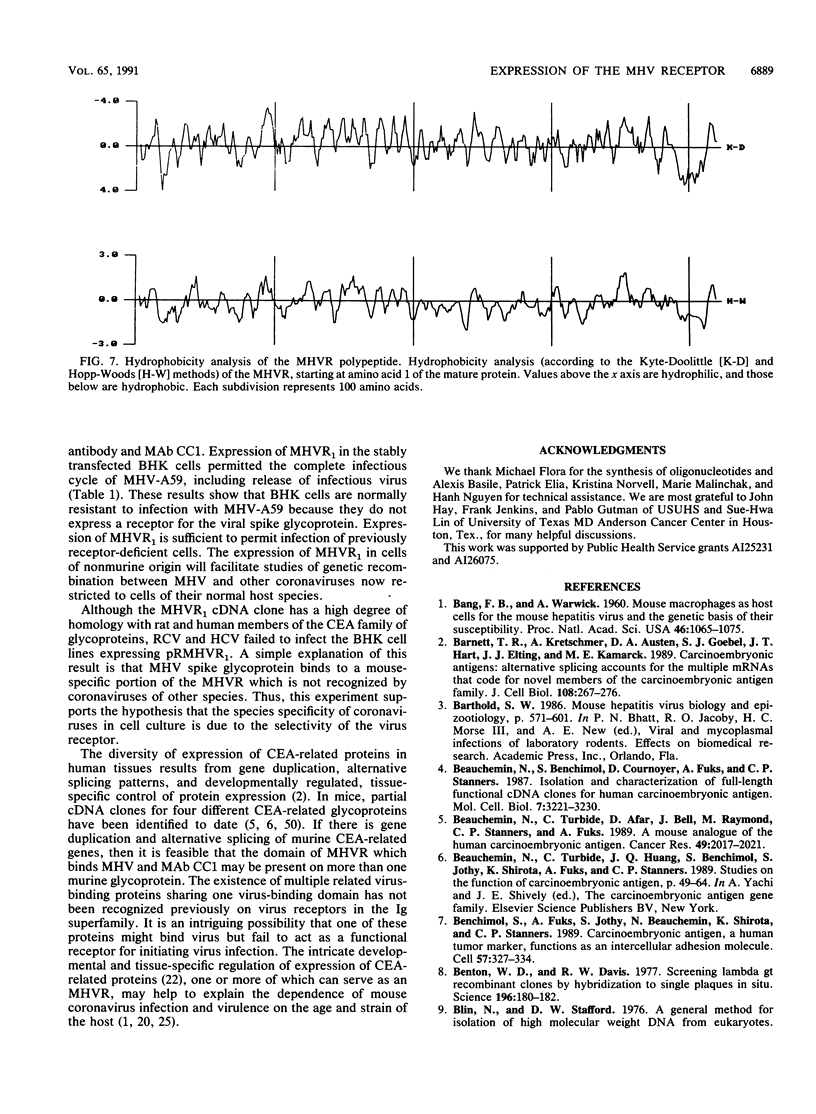
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Q., Turbide C., Daniels E., Jothy S., Beauchemin N. Spatiotemporal expression of murine carcinoembryonic antigen (CEA) gene family members during mouse embryogenesis. Development. 1990 Oct;110(2):573–588. doi: 10.1242/dev.110.2.573. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Tunison L. A., Oldstone M. B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J Gen Virol. 1984 Sep;65(Pt 9):1543–1548. doi: 10.1099/0022-1317-65-9-1543. [DOI] [PubMed] [Google Scholar]
- Kodelja V., Lucas K., Barnert S., von Kleist S., Thompson J. A., Zimmermann W. Identification of a carcinoembryonic antigen gene family in the rat. Analysis of the N-terminal domains reveals immunoglobulin-like, hypervariable regions. J Biol Chem. 1989 Apr 25;264(12):6906–6912. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. The recognition event between virus and host cell receptor: a target for antiviral agents. J Gen Virol. 1990 Apr;71(Pt 4):751–766. doi: 10.1099/0022-1317-71-4-751. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Lowe T., Sharefkin J., Yang S. Q., Dieffenbach C. W. A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Res. 1990 Apr 11;18(7):1757–1761. doi: 10.1093/nar/18.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology. 1988 Oct;166(2):415–422. doi: 10.1016/0042-6822(88)90512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Ryu S. E., Kwong P. D., Truneh A., Porter T. G., Arthos J., Rosenberg M., Dai X. P., Xuong N. H., Axel R., Sweet R. W. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990 Nov 29;348(6300):419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Beatty J. D. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2(4):355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Cardellichio C. B., Winograd D. F., de Souza M. S., Barthold S. W., Holmes K. V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991 Apr;163(4):879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Click R. E., Plagemann P. G. Control of mouse hepatitis virus replication in macrophages by a recessive gene on chromosome 7. J Immunol. 1984 Jul;133(1):428–432. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Ricard C. S., Holmes K. V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J Virol. 1985 Dec;56(3):904–911. doi: 10.1128/jvi.56.3.904-911.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weismiller D. G., Sturman L. S., Buchmeier M. J., Fleming J. O., Holmes K. V. Monoclonal antibodies to the peplomer glycoprotein of coronavirus mouse hepatitis virus identify two subunits and detect a conformational change in the subunit released under mild alkaline conditions. J Virol. 1990 Jun;64(6):3051–3055. doi: 10.1128/jvi.64.6.3051-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M., Littman D. R. Viral receptors of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):725–728. doi: 10.1016/0092-8674(89)90674-0. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
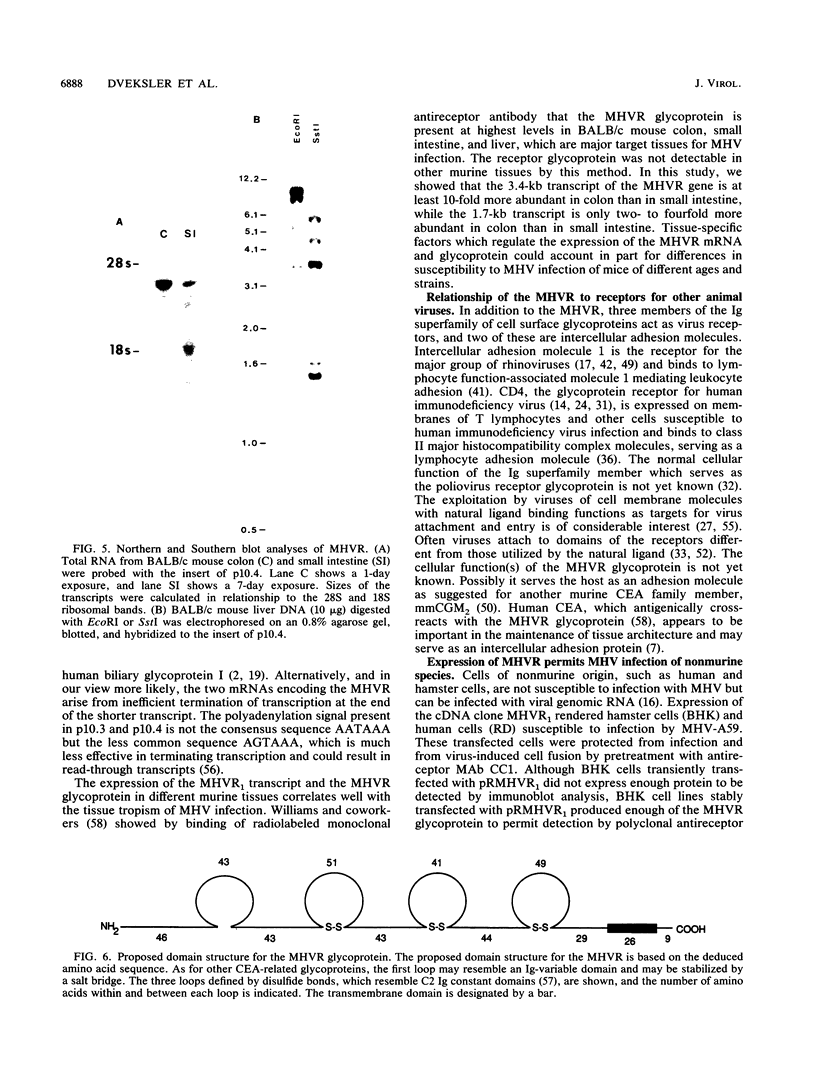
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]