Abstract
The matrix (M) protein of vesicular stomatitis virus serves as an endogenous inhibitor of viral transcription, a function missing or deficient in M proteins of temperature-sensitive (ts) mutants assigned to complementation group III. Previous studies with mutant tsO23(III) and vaccinia virus M-gene expression vectors revealed that the temperature-sensitive phenotype is due to a mutation leading to substitution of phenylalanine for leucine at amino acid III, whereas loss of the major antigenic determinant (epitope 1) of the mutant M protein results from the substitution of glutamic acid for the wild-type amino acid glycine at position 21 (Y. Li, L. Luo, R. M. Snyder, and R. R. Wagner, J. Virol. 62:3729-3737, 1988). We demonstrate here that transcription inhibition activity is restored to rescued tsO23 virus only when the rescuing vaccinia virus recombinant expresses M protein with glycine and not glutamic acid at amino acid 21. These experiments indicate the importance of the conformational integrity of the amino-terminal domain in determining the capacity of the vesicular stomatitis virus M protein to down regulate endogenous transcription.
Full text
PDF
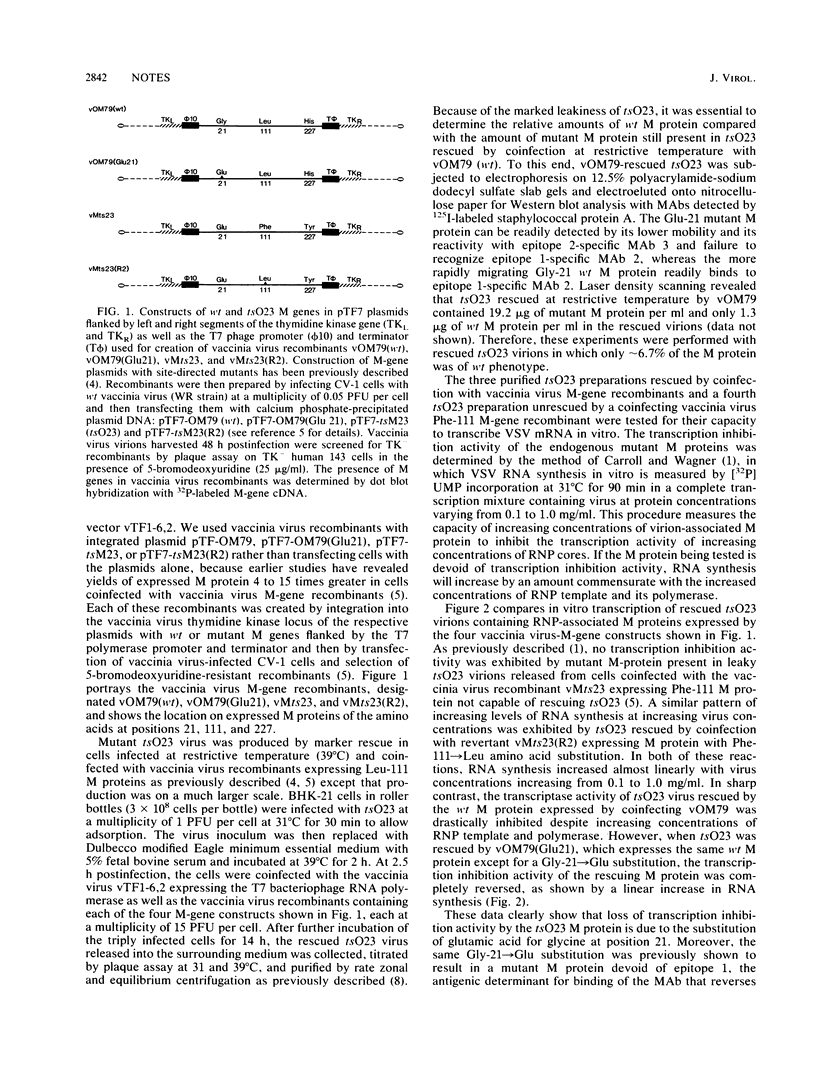
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Expression of the M gene of vesicular stomatitis virus cloned in various vaccinia virus vectors. J Virol. 1988 Mar;62(3):776–782. doi: 10.1128/jvi.62.3.776-782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Site-specific mutations in vectors that express antigenic and temperature-sensitive phenotypes of the M gene of vesicular stomatitis virus. J Virol. 1988 Oct;62(10):3729–3737. doi: 10.1128/jvi.62.10.3729-3737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Vanderoef R., Lenard J. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J Virol. 1987 Feb;61(2):256–263. doi: 10.1128/jvi.61.2.256-263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Lodish H. F. Noninfectious vesicular stomatitis virus particles deficient in the viral nucleocapsid. J Virol. 1979 Feb;29(2):443–447. doi: 10.1128/jvi.29.2.443-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J. B., Pal R., Wagner R. R. Antigenicity, function, and conformation of synthetic oligopeptides corresponding to amino-terminal sequences of wild-type and mutant matrix proteins of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2569–2577. doi: 10.1128/jvi.62.8.2569-2577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Bennett P. L. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. doi: 10.1016/0042-6822(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]


