Abstract
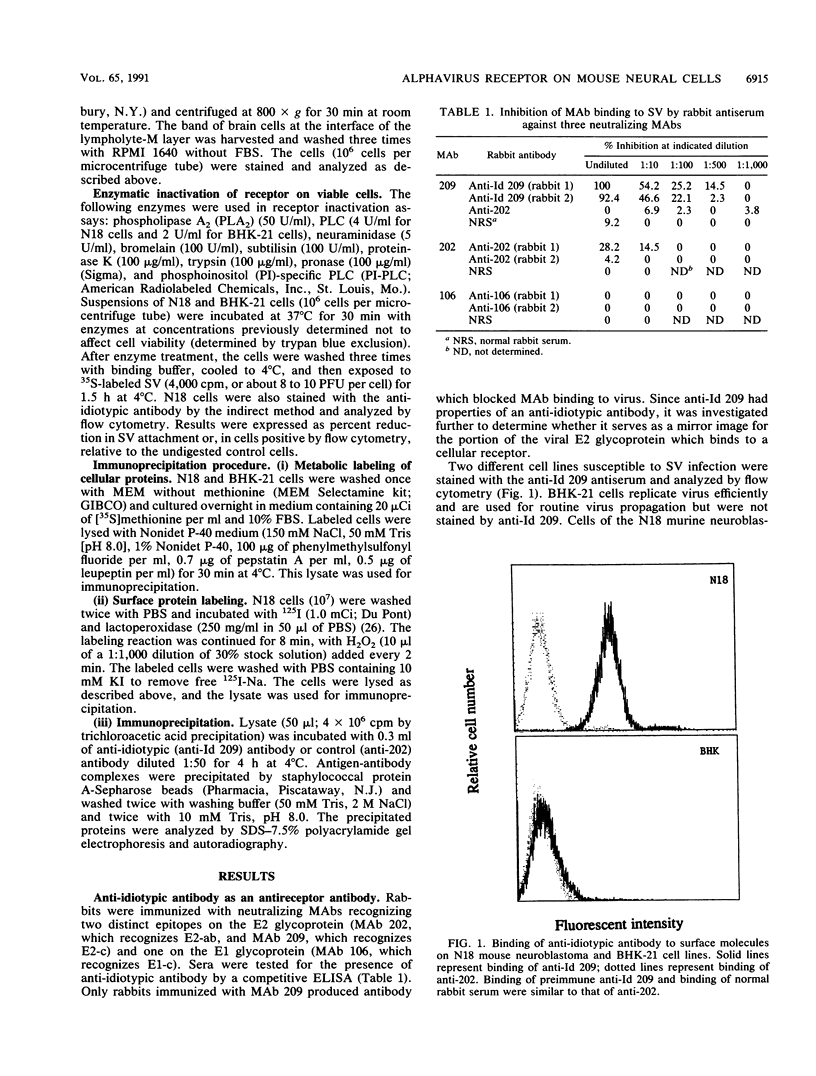
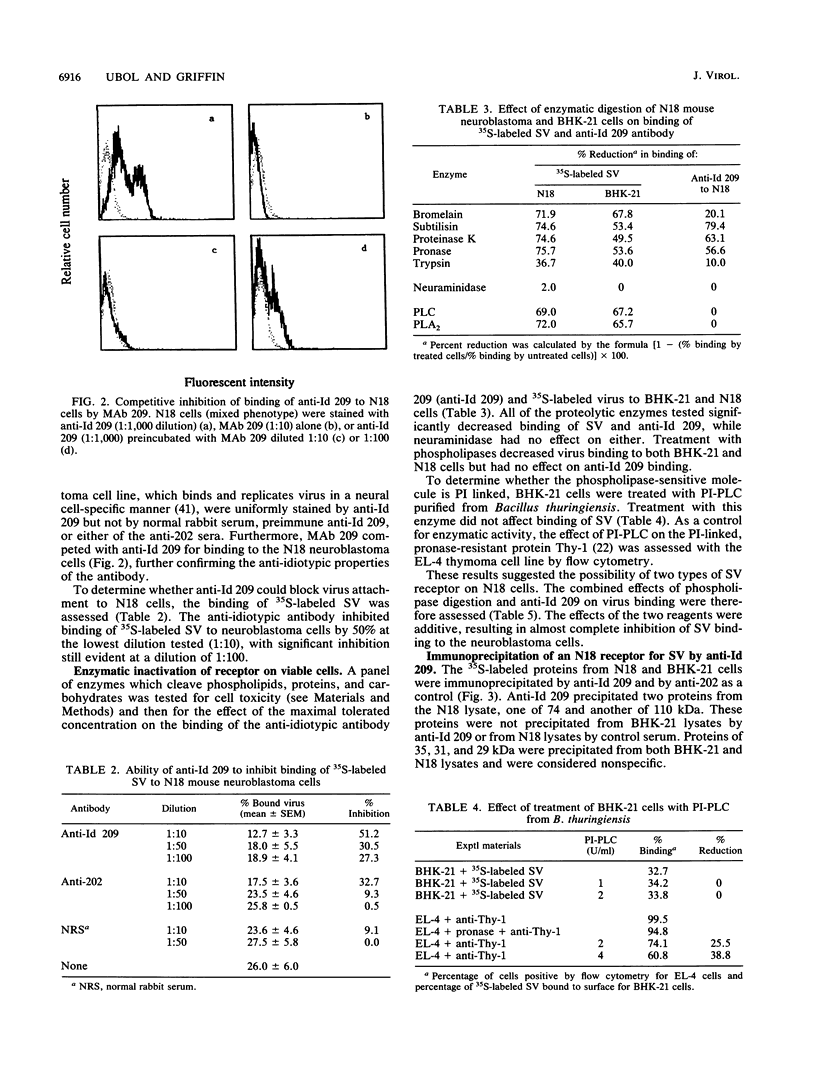
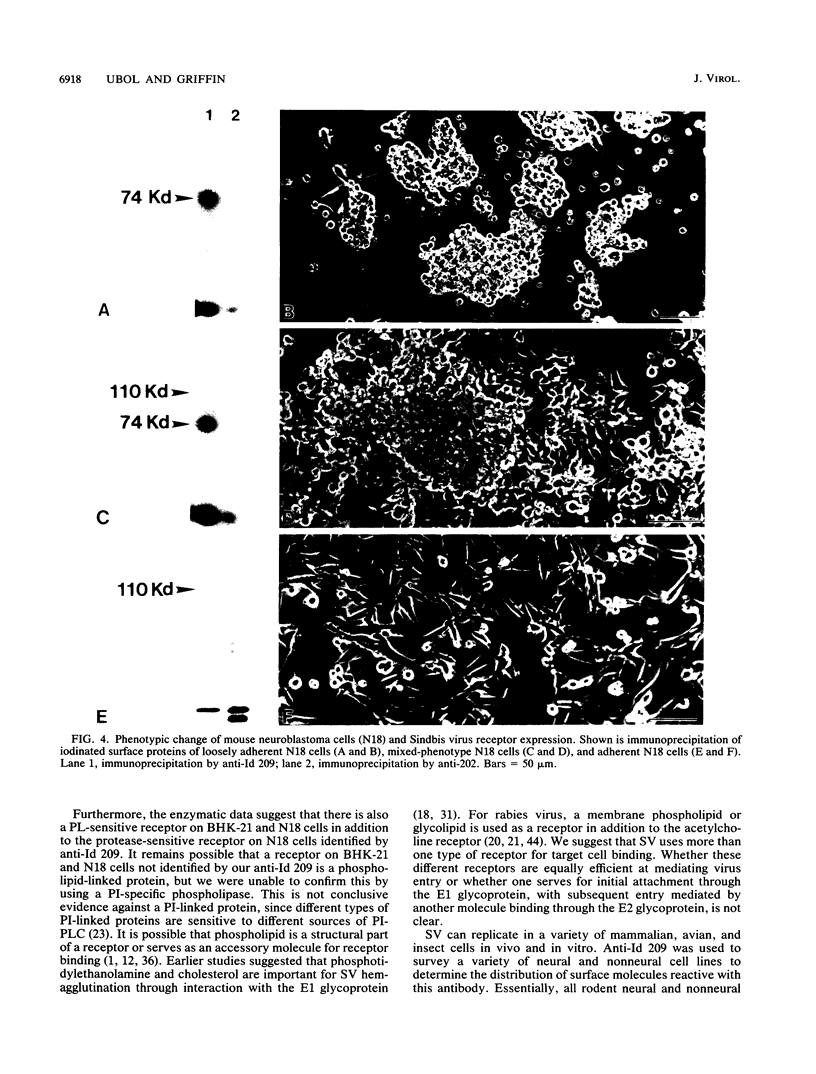
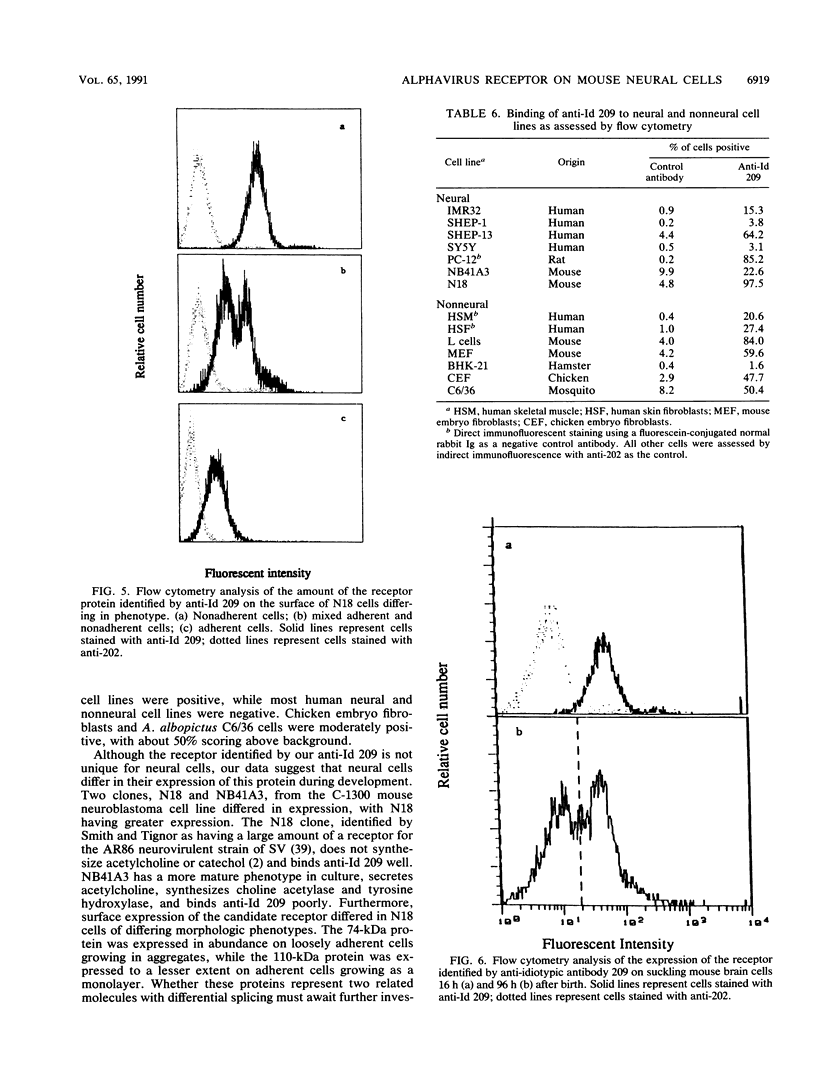
Alphaviruses replicate in a wide variety of cells in vitro. The prototype alphavirus, Sindbis virus, causes an age-dependent encephalitis in mice and serves as an important model system for the study of alphavirus neurovirulence. To begin to understand the role of cellular virus receptors in the pathogenesis of Sindbis virus infection, we developed an anti-idiotypic antibody made in rabbits against a neutralizing monoclonal antibody specific for the E2 surface glycoprotein. The anti-idiotypic antibody (anti-Id 209) bound to N18 mouse neuroblastoma cells and inhibited adsorption of 35S-labeled virus by 50%. Binding of anti-Id 209 was inhibited by pretreatment of N18 cells with various proteases but not with neuraminidase or phospholipase, while virus binding was inhibited by pretreatment with phospholipase as well as protease. Anti-Id 209 precipitated proteins of 110 and 74 kDa from N18 cells intrinsically labeled with [35S]methionine. N18 cells grow with two phenotypes in culture, and immunoprecipitation of 125I-surface-labeled cells showed that the 74-kDa protein was present on loosely adherent cells growing in aggregates, while the 110-kDa protein was present in smaller amounts on firmly adherent cells growing as a monolayer. Analysis of brain cells from newborn mice by flow cytometry showed that all cells expressed the receptor protein at birth, but by 4 days after birth half of the cells had ceased receptor expression. A survey of other cell lines showed the protein to be present on murine fibroblastic and other rodent neuroblastoma cell lines but rarely on human neural or nonneural cell lines. These studies suggest that one of the receptors for Sindbis virus on mouse neural cells is a protein that is regulated during development of the nervous system. Developmental down-regulation of receptor protein expression may contribute to the age-dependent nature of susceptibility of mice to fatal alphavirus encephalitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Itonaga M., Onoue K. Structural studies of Fc receptors. V. Effect of phospholipase C treatment on the binding activities of the Fc receptor of macrophage or its isolated plasma membrane. J Biochem. 1984 Jun;95(6):1593–1602. doi: 10.1093/oxfordjournals.jbchem.a134772. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardman B., Khiroya R. H., Schwartz R. S. Recognition of a leukemia-related antigen by an antiidiotypic antiserum to an anti-gp70 monoclonal antibody. J Exp Med. 1985 Apr 1;161(4):669–686. doi: 10.1084/jem.161.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Tominaga A., Homcy C. J., Fields B. N., Greene M. I. Structural similarities between the mammalian beta-adrenergic and reovirus type 3 receptors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5315–5318. doi: 10.1073/pnas.82.16.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Marsh Y. V., Schreiber A. B., Newman S. R., Todaro G. J., Nestor J. J., Jr Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature. 1985 Dec 19;318(6047):663–665. doi: 10.1038/318663a0. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Dubey L., Steinberg S. P., Sherman D., Gershon M. D., Gershon A. A. Varicella-zoster virus glycoprotein oligosaccharides are phosphorylated during posttranslational maturation. J Virol. 1989 Oct;63(10):4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Griffin D. E. Molecular pathogenesis of Sindbis virus encephalitis in experimental animals. Adv Virus Res. 1989;36:255–271. doi: 10.1016/s0065-3527(08)60587-4. [DOI] [PubMed] [Google Scholar]
- Itonaga M., Aida Y., Onoue K. Structural studies of Fc receptors. IV. Structure required for phospholipids for reconstitution of the delipidated Fc receptor of macrophages. J Biochem. 1984 Apr;95(4):1145–1153. doi: 10.1093/oxfordjournals.jbchem.a134703. [DOI] [PubMed] [Google Scholar]
- Jackson A. C., Moench T. R., Trapp B. D., Griffin D. E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Invest. 1988 May;58(5):503–509. [PubMed] [Google Scholar]
- Johnson R. T., McFarland H. F., Levy S. E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972 Mar;125(3):257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- Kaner R. J., Baird A., Mansukhani A., Basilico C., Summers B. D., Florkiewicz R. Z., Hajjar D. P. Fibroblast growth factor receptor is a portal of cellular entry for herpes simplex virus type 1. Science. 1990 Jun 15;248(4961):1410–1413. doi: 10.1126/science.2162560. [DOI] [PubMed] [Google Scholar]
- Kauffman R. S., Noseworthy J. H., Nepom J. T., Finberg R., Fields B. N., Greene M. I. Cell receptors for the mammalian reovirus. II. Monoclonal anti-idiotypic antibody blocks viral binding to cells. J Immunol. 1983 Nov;131(5):2539–2541. [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koblet H. The "merry-go-round": alphaviruses between vertebrate and invertebrate cells. Adv Virus Res. 1990;38:343–402. doi: 10.1016/s0065-3527(08)60866-0. [DOI] [PubMed] [Google Scholar]
- Kucera P., Dolivo M., Coulon P., Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985 Jul;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988 Jul;62(7):2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen J. A., Terhorst C. Identification of a cell-surface protein involved in the binding site of Sindbis virus on human lymphoblastic cell lines using a heterobifunctional cross-linker. Eur J Biochem. 1981 Mar 16;115(1):153–158. doi: 10.1111/j.1432-1033.1981.tb06211.x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
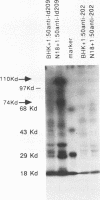
- Marriott S. J., Roeder D. J., Consigli R. A. Anti-idiotypic antibodies to a polyomavirus monoclonal antibody recognize cell surface components of mouse kidney cells and prevent polyomavirus infection. J Virol. 1987 Sep;61(9):2747–2753. doi: 10.1128/jvi.61.9.2747-2753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Mendoza Q. P., Stanley J., Griffin D. E. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J Gen Virol. 1988 Dec;69(Pt 12):3015–3022. doi: 10.1099/0022-1317-69-12-3015. [DOI] [PubMed] [Google Scholar]
- Mooney J. J., Dalrymple J. M., Alving C. R., Russell P. K. Interaction of Sindbis virus with liposomal model membranes. J Virol. 1975 Feb;15(2):225–231. doi: 10.1128/jvi.15.2.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Ogata A., Nagashima K., Hall W. W., Ichikawa M., Kimura-Kuroda J., Yasui K. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J Virol. 1991 Feb;65(2):880–886. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Griffin D. E. Pathogenesis of encephalitis induced in newborn mice by virulent and avirulent strains of Sindbis virus. J Virol. 1990 May;64(5):2041–2046. doi: 10.1128/jvi.64.5.2041-2046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh M. T., Spear P. G. Fibroblast growth factor receptor: does it have a role in the binding of herpes simplex virus? Science. 1991 Jul 12;253(5016):208–210. doi: 10.1126/science.1649495. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Tucker P. C., Griffin D. E. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 Glycoprotein. J Virol. 1991 Mar;65(3):1551–1557. doi: 10.1128/jvi.65.3.1551-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Schmaljohn A. L., Kuhn R. J., Strauss J. H. Antiidiotypic antibodies as probes for the Sindbis virus receptor. Virology. 1991 Apr;181(2):694–702. doi: 10.1016/0042-6822(91)90903-o. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Reagan K. J., Koprowski H. Characterization of saturable binding sites for rabies virus. J Virol. 1984 Jun;50(3):691–697. doi: 10.1128/jvi.50.3.691-697.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]