Abstract
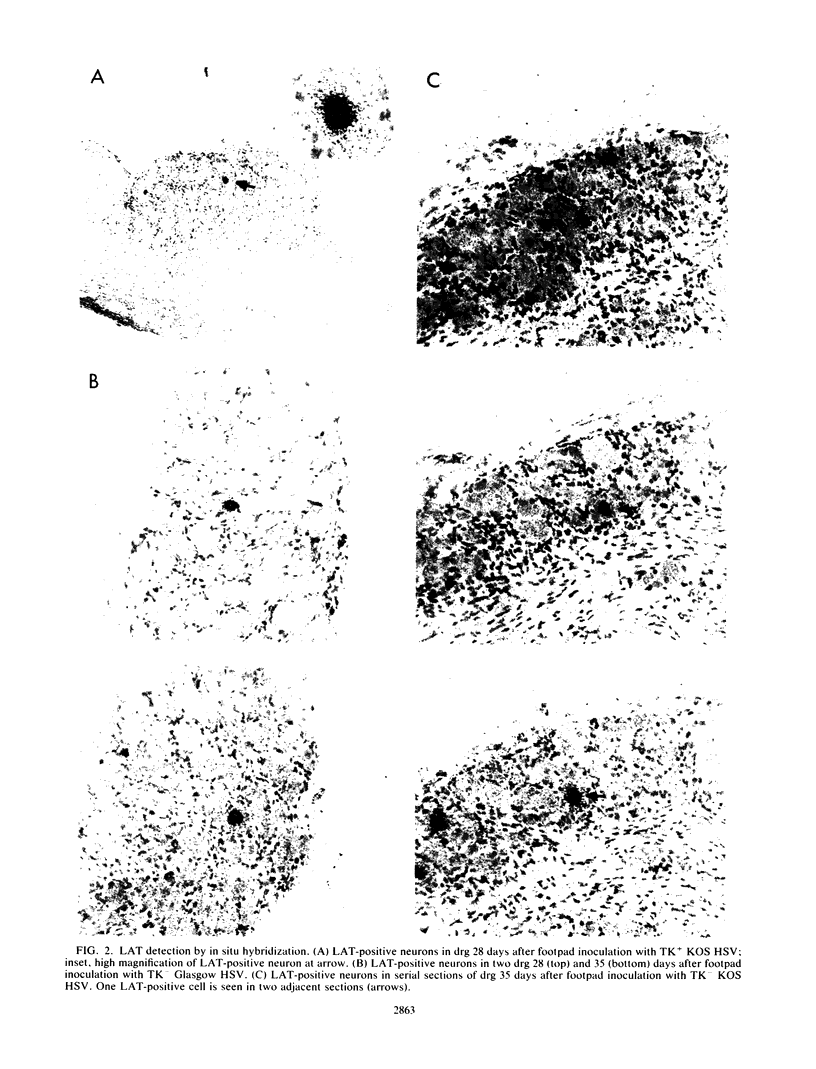
The presence of herpes simplex virus (HSV) latency-associated transcript (LAT) was investigated in sensory ganglion neurons of mice after inoculation with thymidine kinase (TK) mutants of HSV. Ganglion serial sections were examined in order to quantitate numbers of LAT-positive neurons. After inoculation with TK-positive HSV, virus was isolated during latency from explants of most ganglia, and LAT was detected by in situ hybridization in 96% of ganglia. After inoculation with HSV TK mutants, virus was isolated from 0% of ganglia, but LAT was detected in 95 to 100% of ganglia. After inoculation of TK mutants of HSV, therefore, although latent infection as indicated by the isolation of virus from ganglion explants was not detected, the presence of LAT was common. These results suggest that the lack of reactivatable virus after inoculation of HSV TK mutants may be related to a role for HSV TK expression in the reactivation process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Latent herpetic infections following experimental viraemia. J Gen Virol. 1976 Apr;31(1):75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Darby G., Field H. J., Salisbury S. A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981 Jan 1;289(5793):81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Minson A. C., Field H. J., Anderson J. R., Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986 Feb;57(2):446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C. M., McDougall J. K. Limited transcription of the herpes simplex virus genome when latent in human sensory ganglia. J Virol. 1982 Feb;41(2):686–691. doi: 10.1128/jvi.41.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Gilden D. M., Becker Y. HSV-1 thymidine kinase promotes virulence and latency in the mouse. Invest Ophthalmol Vis Sci. 1983 May;24(5):599–602. [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., DeStefano E. Pathogenesis of experimental skin infections induced by drug-resistant herpes simplex virus mutants. Infect Immun. 1981 Dec;34(3):693–701. doi: 10.1128/iai.34.3.693-701.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Coen D. M., Bogard C. L., Hicks K. A., Yager D. R., Knipe D. M., Tyler K. L., Schaffer P. A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989 Feb;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect Immun. 1981 Nov;34(2):571–580. doi: 10.1128/iai.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The organization of the herpes simplex virus genomes. Annu Rev Genet. 1979;13:25–57. doi: 10.1146/annurev.ge.13.120179.000325. [DOI] [PubMed] [Google Scholar]
- Sekizawa T., Openshaw H., Wohlenberg C., Notkins A. L. Latency of herpes simplex virus in absence of neutralizing antibody: model for reactivation. Science. 1980 Nov 28;210(4473):1026–1028. doi: 10.1126/science.6254149. [DOI] [PubMed] [Google Scholar]
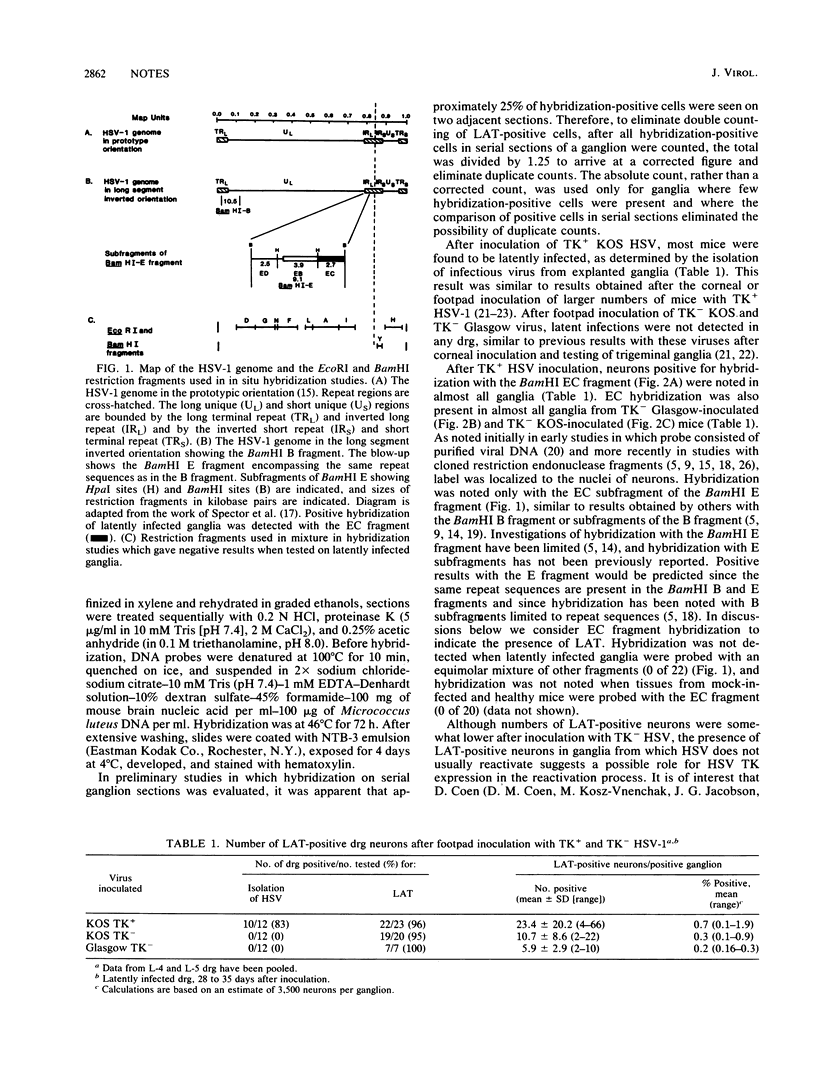
- Spector D. J., Jones T. R., Parks C. L., Deckhut A. M., Hyman R. W. Hybridization between a repeated region of herpes simplex virus type 1 DNA containing the sequence [GGC]n and heterodisperse cellular DNA and RNA. Virus Res. 1987 Feb;7(1):69–82. doi: 10.1016/0168-1702(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988 May;62(5):1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A., Hay K. A. Herpes simplex virus latent infection: reactivation and elimination of latency after neurectomy. Virology. 1988 Nov;167(1):302–305. doi: 10.1016/0042-6822(88)90085-2. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus after in vivo complementation. J Virol. 1987 Jul;61(7):2171–2174. doi: 10.1128/jvi.61.7.2171-2174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Pathogenesis of latent herpes simplex virus infection of the trigeminal ganglion in guinea pigs: effects of age, passive immunization, and hydrocortisone. Infect Immun. 1977 Apr;16(1):69–74. doi: 10.1128/iai.16.1.69-74.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo A. B., Shimeld C., Blyth W. A., Hill T. J., Easty D. L. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982 Nov;63(Pt 1):95–101. doi: 10.1099/0022-1317-63-1-95. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]