Abstract
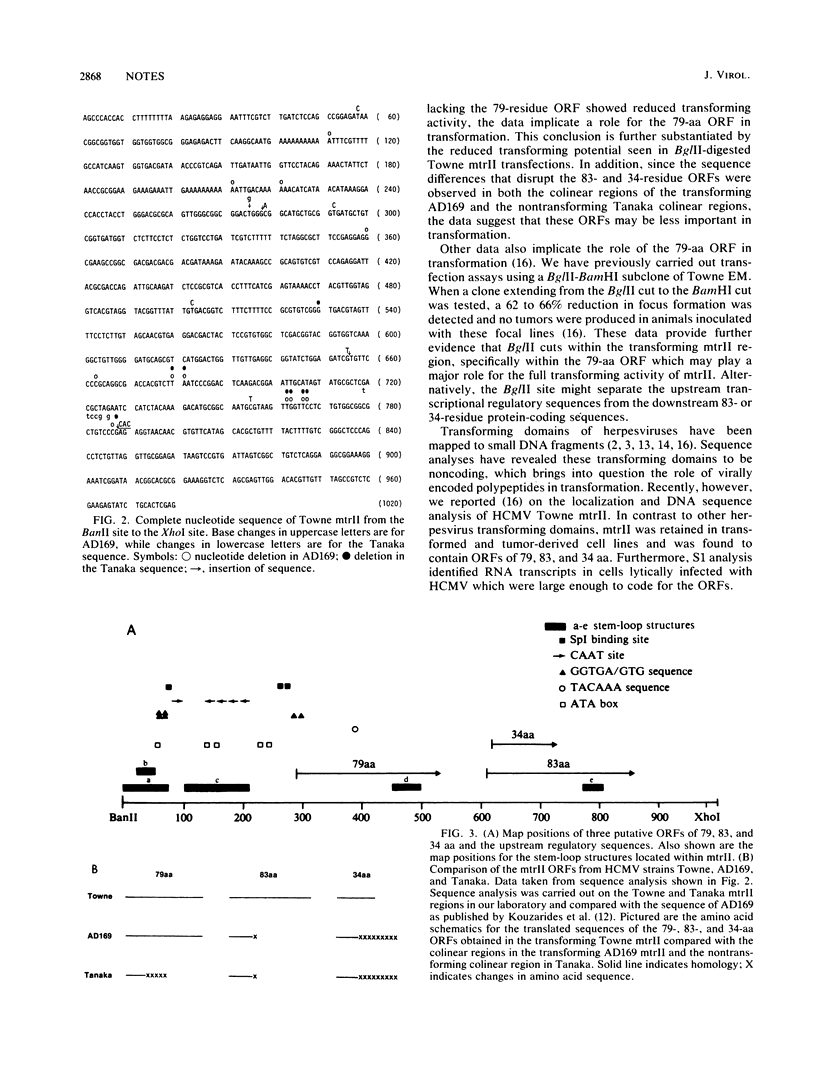
The morphological transforming region II (mtrII) of human cytomegalovirus (HCMV) strain Towne has been localized to a 980-base-pair fragment containing three putative open reading frames (ORFs) of 79, 83, and 34 amino acids (aa). In addition, noncoding DNA sequence elements which have the potential to form stem-loop structures were also observed within mtrII. To determine what elements within HCMV Towne mtrII are important in transformation, colinear regions in other HCMV strains (AD169 and Tanaka) were isolated and a comparison of transforming potential was performed. The results indicated that the 2.2-kilobase colinear region in strain AD169 was transforming, whereas the colinear mtrII region in strain Tanaka showed significantly reduced transforming potential. Analysis of the nucleotide sequence data of these colinear regions revealed the presence of the 79-aa ORF in strains Towne and AD169 and its absence in strain Tanaka. In addition, BglII-digested Towne mtrII, which was cleaved within the 79-aa ORF, was shown to display significantly reduced transforming potential. Since the 83- and 34-aa coding sequences were interrupted in both the transforming AD169 colinear region and the nontransforming Tanaka strains, these ORFs were thought not to be important in transformation. Analysis of the stem-loop structures within each of the mtrII colinear regions did not reveal significant changes among the transforming and nontransforming colinear fragments. Thus, the comparative data indicate an important role for the 79-aa ORF in transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- Clanton D. J., Jariwalla R. J., Kress C., Rosenthal L. J. Neoplastic transformation by a cloned human cytomegalovirus DNA fragment uniquely homologous to one of the transforming regions of herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3826–3830. doi: 10.1073/pnas.80.12.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Nelson J. A., McDougall J. K. Small fragments of herpesvirus DNA with transforming activity contain insertion sequence-like structures. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4736–4740. doi: 10.1073/pnas.81.15.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder L., Sanford E. J., Rohner T. J., Rapp F. Cytomegalovirus and cancer of the prostate: in vitro transformation of human cells. Cancer Treat Rep. 1977 Mar-Apr;61(2):139–146. [PubMed] [Google Scholar]
- Giraldo G., Beth E., Henle W., Henle G., Mike V., Safai B., Huraux J. M., McHardy J., deThé G. Antibody patterns to herpesviruses in Kaposi's sarcoma. II. Serological association of American Kaposi's sarcoma with cytomegalovirus. Int J Cancer. 1978 Aug 15;22(2):126–131. doi: 10.1002/ijc.2910220204. [DOI] [PubMed] [Google Scholar]
- Giraldo G., Beth E., Huang E. S. Kaposi's sarcoma and its relationship to cytomegalovirus (CMNV). III. CMV DNA and CMV early antigens in Kaposi's sarcoma. Int J Cancer. 1980 Jul 15;26(1):23–29. doi: 10.1002/ijc.2910260105. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Roche J. K. Cytomegalovirus D.N.A. and adenocarcinoma of the colon: Evidence for latent viral infection. Lancet. 1978 May 6;1(8071):957–960. doi: 10.1016/s0140-6736(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Immortalization and neoplastic transformation of normal diploid cells by defined cloned DNA fragments of herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5902–5906. doi: 10.1073/pnas.80.19.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Razzaque A., Lawson S., Rosenthal L. J. Tumor progression mediated by two cooperating DNA segments of human cytomegalovirus. J Virol. 1989 Jan;63(1):425–428. doi: 10.1128/jvi.63.1.425-428.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Barrell B. G. Nucleotide sequence of the transforming region of human cytomegalovirus. Mol Biol Med. 1983 Jul;1(1):47–58. [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Galloway D. A., McDougall J. K. Transformation of NIH 3T3 cells with cloned fragments of human cytomegalovirus strain AD169. J Virol. 1982 Jul;43(1):83–91. doi: 10.1128/jvi.43.1.83-91.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Jahn G., Galloway D. A., McDougall J. K. Structure of the transforming region of human cytomegalovirus AD169. J Virol. 1984 Jan;49(1):109–115. doi: 10.1128/jvi.49.1.109-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacsa A. S., Kummerländer L., Pejtsik B., Pali K. Herpesvirus antibodies and antigens in patients with cervical anaplasia and in controls. J Natl Cancer Inst. 1975 Oct;55(4):775–781. doi: 10.1093/jnci/55.4.775. [DOI] [PubMed] [Google Scholar]
- Razzaque A., Jahan N., McWeeney D., Jariwalla R. J., Jones C., Brady J., Rosenthal L. J. Localization and DNA sequence analysis of the transforming domain (mtrII) of human cytomegalovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5709–5713. doi: 10.1073/pnas.85.15.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Takekoshi M., Ihara S., Tanaka S., Maeda-Takekoshi F., Watanabe Y. A new human cytomegalovirus isolate has an invertible subsegment within its L component producing eight genome isomers. J Gen Virol. 1987 Mar;68(Pt 3):765–776. doi: 10.1099/0022-1317-68-3-765. [DOI] [PubMed] [Google Scholar]
- el-Beik T., Razzaque A., Jariwalla R., Cihlar R. L., Rosenthal L. J. Multiple transforming regions of human cytomegalovirus DNA. J Virol. 1986 Nov;60(2):645–652. doi: 10.1128/jvi.60.2.645-652.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



