Abstract
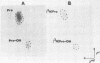
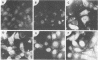
Amino acid analysis of [3H]proline-labeled polyomavirus major capsid protein VP1 by two-dimensional paper chromatography of the acid-hydrolyzed protein revealed the presence of 3H-labeled hydroxyproline. Addition of the proline analog L-azetidine-2-carboxylic acid to infected mouse kidney cell cultures prevented or greatly reduced hydroxylation of proline in VP1. Immunofluorescence analysis performed on infected cells over a time course of analog addition revealed that virus proteins were synthesized but that transport from the cytoplasm to the nucleus was impeded. A reduction in the assembly of progeny virions demonstrated by CsCl gradient purification of virus from [35S]methionine-labeled infected cell cultures was found to correlate with the time of analog addition. These results suggest that incorporation of this proline analog into VP1, accompanied by reduction of the hydroxyproline content of the protein, influences the amount of virus progeny produced by affecting transport of VP1 to the cell nucleus for assembly into virus particles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Consigli R. A. Chemical cleavage of polyomavirus major structural protein VP1: identification of cleavage products and evidence that the receptor moiety resides in the carboxy-terminal region. J Virol. 1983 Oct;48(1):197–205. doi: 10.1128/jvi.48.1.197-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Consigli R. A. Comparison of nonphosphorylated and phosphorylated species of polyomavirus major capsid protein VP1 and identification of the major phosphorylation region. J Virol. 1983 Oct;48(1):206–217. doi: 10.1128/jvi.48.1.206-217.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji V. N., Rao M. J., Rao S. N., Dietrich S. W., Sasisekharan V. Geometry of proline and hydroxyproline I: An analysis of X-ray crystal structure data. Biochem Biophys Res Commun. 1986 Nov 14;140(3):895–900. doi: 10.1016/0006-291x(86)90719-9. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Anders D. G., Trempy J., Consigli R. A. Differences in the subpopulations of the structural proteins of polyoma virions and capsids: biological functions of the multiple VP1 species. J Virol. 1981 Jan;37(1):80–91. doi: 10.1128/jvi.37.1.80-91.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Kendall J. D., Consigli R. A. In vitro reassembly of infectious polyoma virions. J Virol. 1979 Nov;32(2):640–647. doi: 10.1128/jvi.32.2.640-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Decapsidation of polyoma virus: identification of subviral species. Virology. 1975 Nov;68(1):245–255. doi: 10.1016/0042-6822(75)90165-8. [DOI] [PubMed] [Google Scholar]
- Garcea R. L., Ballmer-Hofer K., Benjamin T. L. Virion assembly defect of polyomavirus hr-t mutants: underphosphorylation of major capsid protein VP1 before viral DNA encapsidation. J Virol. 1985 May;54(2):311–316. doi: 10.1128/jvi.54.2.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Jimenez S., Rosenbloom J. Decreased thermal stability of collagens containing analogs of proline or lysine. Arch Biochem Biophys. 1974 Aug;163(2):459–465. doi: 10.1016/0003-9861(74)90502-5. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Flory P. J., Jr Synthesis of the SV40 viral polypeptide Vp1 during infection. Virology. 1978 May 15;86(2):344–353. doi: 10.1016/0042-6822(78)90075-2. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S., Felix A. M. Effect of L-3,4-dehydroproline on collagen synthesis and prolyl hydroxylase activity in mammalian cell cultures. J Biol Chem. 1976 Jan 25;251(2):503–509. [PubMed] [Google Scholar]
- Ludlow J. W., Consigli R. A. Differences in biological activity and structural protein VP1 phosphorylation of polyomavirus progeny resulting from infection of primary mouse kidney and primary mouse embryo cell cultures. J Virol. 1987 Feb;61(2):509–515. doi: 10.1128/jvi.61.2.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Consigli R. A. Localization of calcium on the polyomavirus VP1 capsid protein. J Virol. 1987 Sep;61(9):2934–2937. doi: 10.1128/jvi.61.9.2934-2937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Consigli R. A. Polyomavirus major capsid protein VP1 is modified by tyrosine sulfuration. J Virol. 1987 May;61(5):1708–1711. doi: 10.1128/jvi.61.5.1708-1711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Consigli R. A. Production and characterization of monoclonal antibodies to polyomavirus major capsid protein VP1. J Virol. 1985 Nov;56(2):365–372. doi: 10.1128/jvi.56.2.365-372.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Robbins A. K., Crawford L. V. Phophorylation of polyoma and SV40 virus proteins. J Gen Virol. 1977 Oct;37(1):75–83. doi: 10.1099/0022-1317-37-1-75. [DOI] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Generation of capsids from unstable polyoma virions. J Virol. 1983 Sep;47(3):620–625. doi: 10.1128/jvi.47.3.620-625.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Improved infectivity of reassembled polyoma virus. J Virol. 1982 Jul;43(1):337–341. doi: 10.1128/jvi.43.1.337-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]