Abstract
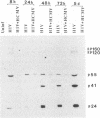
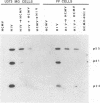
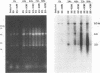
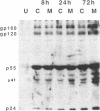
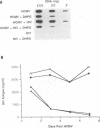
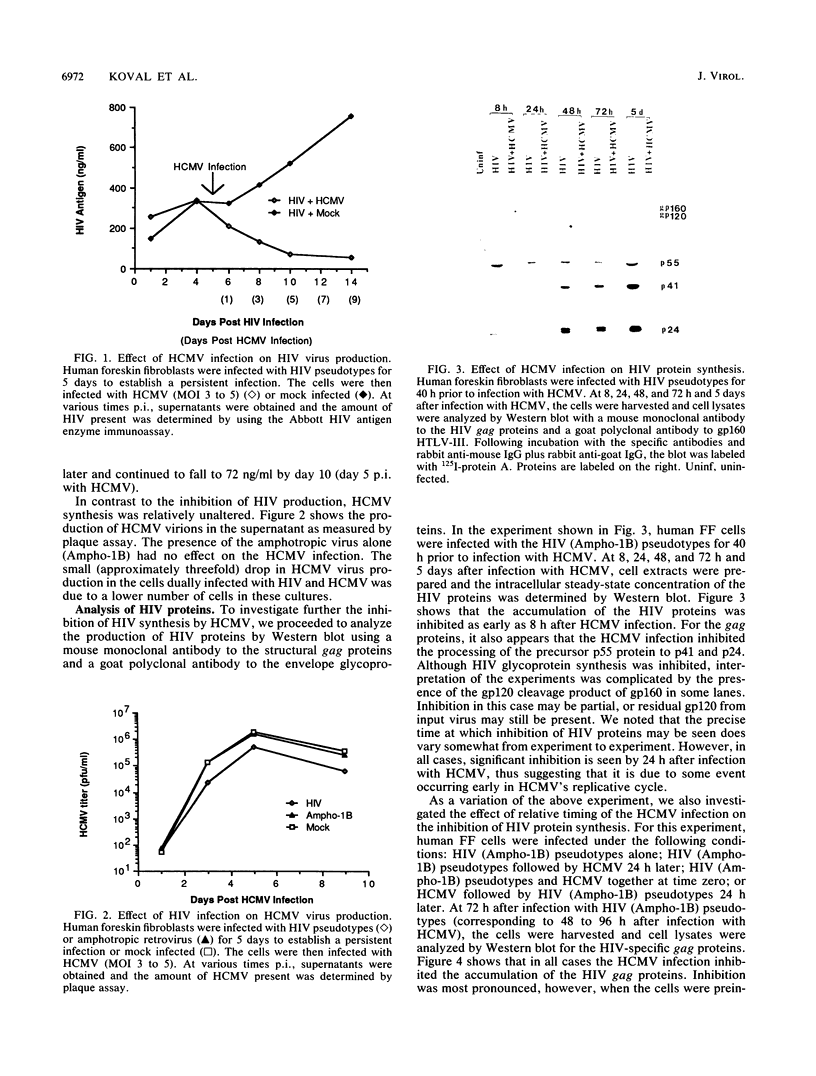
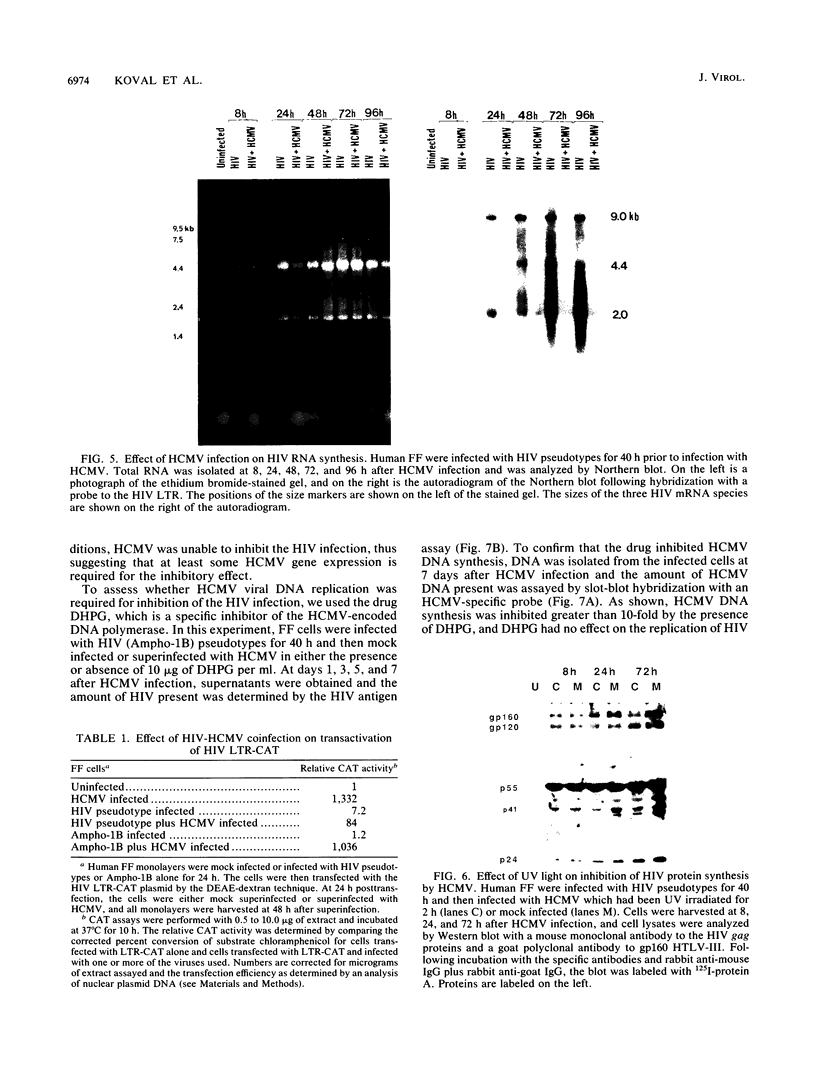
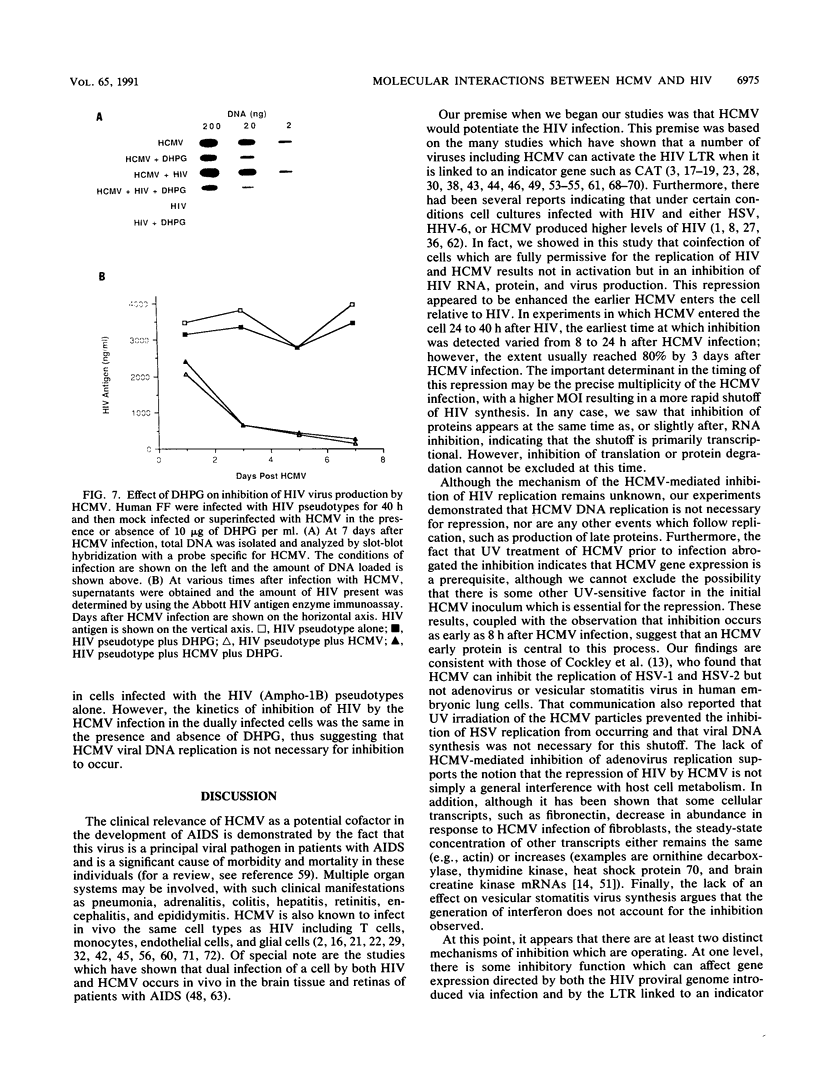
We have been studying the role of human cytomegalovirus (HCMV) as a potential cofactor in human immunodeficiency virus (HIV)-related disease. The clinical relevance of HCMV is highlighted by the fact that it is a principal viral pathogen in patients with AIDS and is known to infect the same cells as HIV. In this study, we focused on the molecular interactions between HIV and HCMV in human fibroblasts and in the human glioblastoma/astrocytoma-derived cell line U373 MG, cells which can be productively infected by both viruses. Because these cells are CD4-, we used HIV pseudotyped with a murine amphotropic retrovirus as described previously (D. H. Spector, E. Wade, D. A. Wright, V. Koval, C. Clark, D. Jaquish, and S. A. Spector, J. Virol. 64:2298-2308, 1990). Initial studies showed that when cells were preinfected with HIV (Ampho-1B) for 5 days and then superinfected with HCMV, HIV antigen production dropped significantly in the coinfected cells but continued to rise in cells infected with HIV (Ampho-1B) alone. HCMV production, however, was unaffected by the presence of HIV. Further analysis showed that HIV steady-state RNA levels and gag and env protein production were also inhibited in the presence of HCMV. The transcriptional inhibition of HIV was particularly surprising in view of the previous results of several other laboratories as well as our own that HCMV infection stimulates HIV long terminal repeat-chloramphenicol acetyltransferase (LTR-CAT) expression in transient expression assays. To investigate this further, we transfected the HIV LTR-CAT construct into either uninfected cells or cells which had been preinfected with HIV. The cells were infected with HCMV 24 h posttransfection and assayed for CAT gene expression at 48 h after HCMV infection. Although there was some stimulation of the LTR-CAT in cells that were dually infected by HIV and HCMV, it was 16-fold less than that in the cells infected only with HCMV. This suggests that in the presence of the HIV infection, the stimulation of the HIV LTR-CAT gene by HCMV is significantly reduced. Experiments with UV-irradiated HCMV and the HCMV DNA polymerase inhibitor ganciclovir showed that HCMV transcription is necessary for the reduction in HIV production to occur; however, replication of the HCMV genome or any events which take place after DNA replication are not necessary. These results, coupled with the observation that inhibition is usually first seen between 8 and 24 h after HCMV infection, suggest that an HCMV early protein is involved in repression of HIV.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht M. A., DeLuca N. A., Byrn R. A., Schaffer P. A., Hammer S. M. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J Virol. 1989 May;63(5):1861–1868. doi: 10.1128/jvi.63.5.1861-1868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. A., Pratt-Lowe E., Peterlin B. M., Luciw P. A. Cytomegalovirus activates transcription directed by the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2932–2940. doi: 10.1128/jvi.64.6.2932-2940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Animal virus pseudotypes. Prog Med Virol. 1979;25:37–68. [PubMed] [Google Scholar]
- Canivet M., Hoffman A. D., Hardy D., Sernatinger J., Levy J. A. Replication of HIV-1 in a wide variety of animal cells following phenotypic mixing with murine retroviruses. Virology. 1990 Oct;178(2):543–551. doi: 10.1016/0042-6822(90)90352-r. [DOI] [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K., Tapper M. A. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J Infect Dis. 1990 Oct;162(4):844–851. doi: 10.1093/infdis/162.4.844. [DOI] [PubMed] [Google Scholar]
- Casareale D., Fiala M., Chang C. M., Cone L. A., Mocarski E. S. Cytomegalovirus enhances lysis of HIV-infected T lymphoblasts. Int J Cancer. 1989 Jul 15;44(1):124–130. doi: 10.1002/ijc.2910440122. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Buller R., Portis J., Wehrly K. Failure of human immunodeficiency virus entry and infection in CD4-positive human brain and skin cells. J Virol. 1990 Jan;64(1):215–221. doi: 10.1128/jvi.64.1.215-221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Maury W. Differential expression in human and mouse cells of human immunodeficiency virus pseudotyped by murine retroviruses. J Virol. 1990 Sep;64(9):4553–4557. doi: 10.1128/jvi.64.9.4553-4557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K. A., Robbins P. B., Fernie B., Ostrove J. M., Fauci A. S. Viral antigen stimulation of the production of human monokines capable of regulating HIV1 expression. J Immunol. 1989 Jul 15;143(2):470–475. [PubMed] [Google Scholar]
- Cockley K. D., Shiraki K., Rapp F. A human cytomegalovirus function inhibits replication of herpes simplex virus. J Virol. 1988 Jan;62(1):188–195. doi: 10.1128/jvi.62.1.188-195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Santomenna L. D. Selective induction of chromosomal gene expression by human cytomegalovirus. Virology. 1988 Sep;166(1):217–228. doi: 10.1016/0042-6822(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991 Mar;65(3):1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner W. M., McCutchan J. A., Richman D. D., Hirata K., Spector S. A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990 Jan;161(1):31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos H., Elfassi E., Michelson S., Arenzana-Seisdedos F., Hazan U., Munier A., Virelizier J. L. Cytomegalovirus infection and trans-activation of HIV-1 and HIV-2 LTRs in human astrocytoma cells. AIDS Res Hum Retroviruses. 1989 Apr;5(2):217–224. doi: 10.1089/aid.1989.5.217. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D. H., Ho D. D., de la Monte S. M., Hirsch M. S., Rota T. R., Sobel R. A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986 Sep;20(3):289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. E., Yang J. Y., Zhang R. D., Bealer M. Altered HIV expression and EBV-induced transformation in coinfected PBLs and PBL subpopulations. Virology. 1991 May;182(1):186–198. doi: 10.1016/0042-6822(91)90662-u. [DOI] [PubMed] [Google Scholar]
- Hirai K., Maeda F., Watanabe Y. Expression of early virus functions in human cytomegalovirus infected HEL cells: effect of ultraviolet light-irradiation of the virus. J Gen Virol. 1978 Jan;38(1):121–133. doi: 10.1099/0022-1317-38-1-121. [DOI] [PubMed] [Google Scholar]
- Ho W. Z., Harouse J. M., Rando R. F., Gönczöl E., Srinivasan A., Plotkin S. A. Reciprocal enhancement of gene expression and viral replication between human cytomegalovirus and human immunodeficiency virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):97–103. doi: 10.1099/0022-1317-71-1-97. [DOI] [PubMed] [Google Scholar]
- Horvat R. T., Wood C., Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. doi: 10.1128/jvi.63.2.970-973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keh W. C., Gerber M. A. In situ hybridization for cytomegalovirus DNA in AIDS patients. Am J Pathol. 1988 Jun;131(3):490–496. [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Page K. A., Littman D. R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991 Jan;65(1):162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Landay A., Lennette E. T. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J Clin Microbiol. 1990 Oct;28(10):2362–2364. doi: 10.1128/jcm.28.10.2362-2364.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Lori F., Gallo R. C. CD4-independent infection by human immunodeficiency virus type 1 after phenotypic mixing with human T-cell leukemia viruses. J Virol. 1990 Dec;64(12):6341–6344. doi: 10.1128/jvi.64.12.6341-6344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., di Marzo Veronese F., Ensoli B., Franchini G., Jemma C., DeRocco S. E., Kalyanaraman V. S., Gallo R. C. Expanded HIV-1 cellular tropism by phenotypic mixing with murine endogenous retroviruses. Science. 1990 Feb 16;247(4944):848–852. doi: 10.1126/science.2305256. [DOI] [PubMed] [Google Scholar]
- Mallon R., Borkowski J., Albin R., Pepitoni S., Schwartz J., Kieff E. The Epstein-Barr virus BZLF1 gene product activates the human immunodeficiency virus type 1 5' long terminal repeat. J Virol. 1990 Dec;64(12):6282–6285. doi: 10.1128/jvi.64.12.6282-6285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. R., Spector D. H. Replication of the murine cytomegalovirus genome: structure and role of the termini in the generation and cleavage of concatenates. Virology. 1988 Jan;162(1):98–107. doi: 10.1016/0042-6822(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Weiss R. A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990 Feb 15;343(6259):659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- Morgello S., Cho E. S., Nielsen S., Devinsky O., Petito C. K. Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of the literature. Hum Pathol. 1987 Mar;18(3):289–297. doi: 10.1016/s0046-8177(87)80012-6. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Myerson D., Hackman R. C., Nelson J. A., Ward D. C., McDougall J. K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Ghazal P., Wiley C. A. Role of opportunistic viral infections in AIDS. AIDS. 1990 Jan;4(1):1–10. doi: 10.1097/00002030-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Reynolds-Kohler C., Oldstone M. B., Wiley C. A. HIV and HCMV coinfect brain cells in patients with AIDS. Virology. 1988 Jul;165(1):286–290. doi: 10.1016/0042-6822(88)90685-x. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. A., Landau N. R., Littman D. R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990 Nov;64(11):5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande H., Terramani T., Tressel T., Churchill M. A., Hawkins G. G., Zaia J. A. Altered expression of fibronectin gene in cells infected with human cytomegalovirus. J Virol. 1990 Mar;64(3):1366–1369. doi: 10.1128/jvi.64.3.1366-1369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Rando R. F., Srinivasan A., Feingold J., Gonczol E., Plotkin S. Characterization of multiple molecular interactions between human cytomegalovirus (HCMV) and human immunodeficiency virus type 1 (HIV-1). Virology. 1990 May;176(1):87–97. doi: 10.1016/0042-6822(90)90233-h. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Trans-activation of the human immunodeficiency virus long terminal repeat sequences, expressed in an adenovirus vector, by the adenovirus E1A 13S protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4200–4204. doi: 10.1073/pnas.85.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Lynn D. L., Robert-Guroff M., Langlois A. J., Lyerly H. K., Carson H., Krohn K., Ranki A., Gallo R. C., Bolognesi D. P. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6924–6928. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooley R. T. Cytomegalovirus in the setting of infection with human immunodeficiency virus. Rev Infect Dis. 1990 Sep-Oct;12 (Suppl 7):S811–S819. doi: 10.1093/clinids/12.supplement_7.s811. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik P. R., Kosloff B. R., Hirsch M. S. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988 Mar;157(3):508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- Skolnik P. R., Pomerantz R. J., de la Monte S. M., Lee S. F., Hsiung G. D., Foos R. Y., Cowan G. M., Kosloff B. R., Hirsch M. S., Pepose J. S. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol. 1989 Apr 15;107(4):361–372. doi: 10.1016/0002-9394(89)90659-4. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Wade E., Wright D. A., Koval V., Clark C., Jaquish D., Spector S. A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990 May;64(5):2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Rabert D. K., Spector D. H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988 Sep;62(9):3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G. The Epstein-Barr virus and the immune system. Adv Cancer Res. 1987;49:75–125. doi: 10.1016/s0065-230x(08)60795-2. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Robinson W. S. Hepatitis B virus X gene can transactivate heterologous viral sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2046–2050. doi: 10.1073/pnas.86.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Rosen C. A., Haseltine W. A., Robinson W. S. Identification of a region within the human immunodeficiency virus type 1 long terminal repeat that is essential for transactivation by the hepatitis B virus gene X. J Virol. 1989 Jun;63(6):2857–2860. doi: 10.1128/jvi.63.6.2857-2860.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Nelson J. A. Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am J Pathol. 1988 Oct;133(1):73–81. [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Denaro F. J., Nelson J. A., Lampert P. W., Oldstone M. B. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J Neuropathol Exp Neurol. 1986 Mar;45(2):127–139. doi: 10.1097/00005072-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Zhu Z. H., Chen S. S., Huang A. S. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3(3):215–219. [PubMed] [Google Scholar]
- Závada J. Viral pseudotypes and phenotypic mixing. Arch Virol. 1976;50(1-2):1–15. doi: 10.1007/BF01317996. [DOI] [PubMed] [Google Scholar]