Abstract
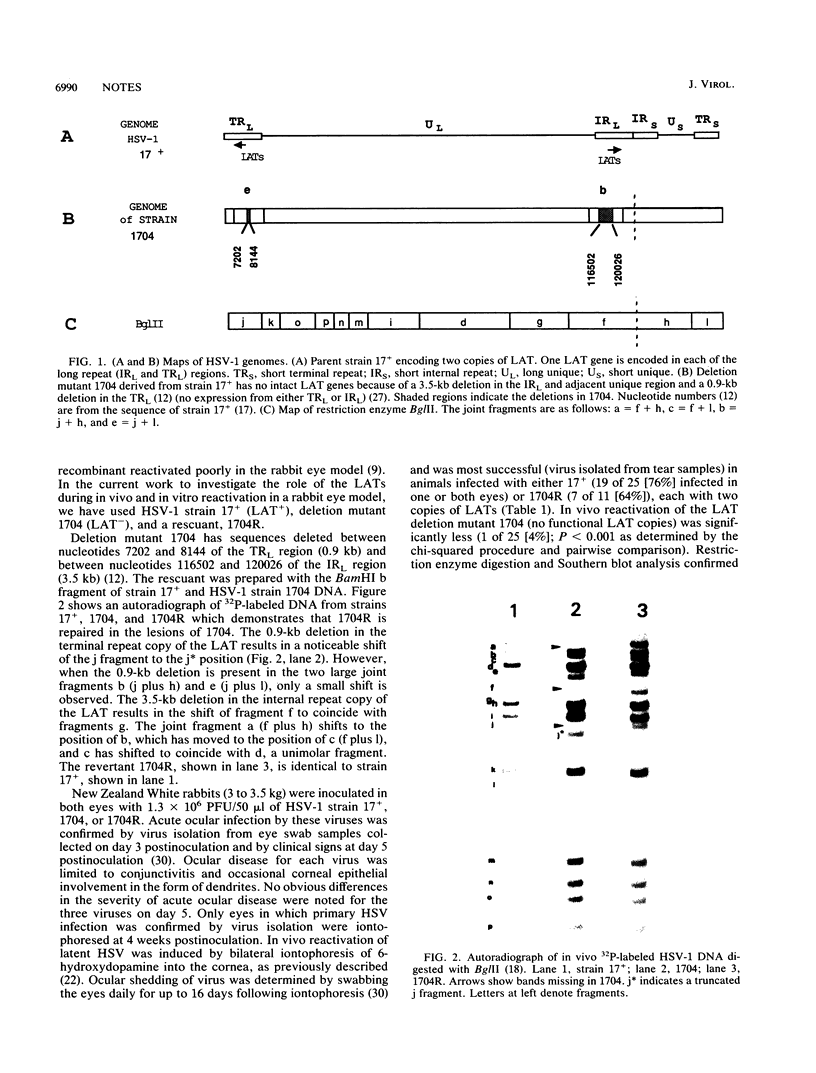
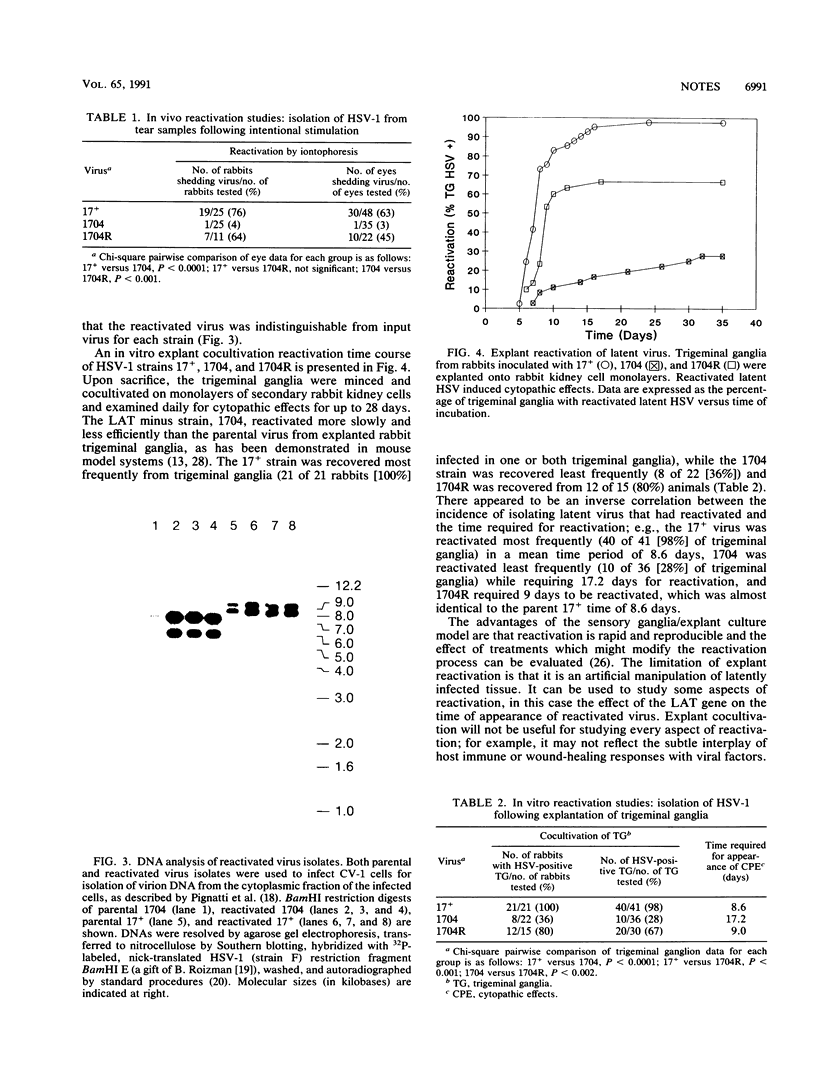
Many recent studies of latent herpes simplex virus type 1 (HSV-1) infections within the nervous system have focused on the diploid genes encoding the latency-associated transcripts (LATs). The impaired explant reactivation of LAT variants from mouse trigeminal ganglia has implicated the LATs in the efficiency or speed of the reactivation process (D. A. Leib, C. L. Bogard, M. Kosz-Vnenchak, K. A. Hicks, D. M. Coen, D. M. Knipe, and P. A. Schaffer, J. Virol. 63:2893-2900, 1989; I. Steiner, J. G. Spivack, R. P. Lirette, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser, EMBO J. 8:505-511, 1989). However, it is not known how closely explant reactivation mimics the reactivation process in vivo. In the current study, a LAT variant (1704), parental strain (17+), and rescuant (1704R) were compared in vivo for reactivation of latent infection by iontophoresis in the rabbit eye model and in vitro by explant cocultivation of trigeminal ganglia from rabbits. Following iontophoresis, 17+ and 1704R reactivated in vivo from 76 and 64% of rabbits, respectively, while 1704 reactivated only from 4% (1 of 25) of the animals. In explant reactivation experiments, 17+ and 1704R reactivated from 98 and 67% of rabbit trigeminal ganglia, while 1704 reactivated from only 28% of trigeminal ganglia. The mean time required for the appearance of reactivated 1704 in explant culture, 17 days, was significantly longer than for 17+ and 1704R, 8 to 9 days. Thus, the explant reactivation kinetics in rabbit trigeminal ganglia reflect the behavior of LAT variant 1704 in vivo in the rabbit eye model. These data support the role of the LATs in the reactivation process and support the hypothesis that explant reactivation is a suitable system for analyzing the biological behavior of HSV-1 variants with defined genetic alterations in the LAT gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. J., Dobson A. T., Feldman L. T. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):790–794. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Spivack J. G., Wroblewska Z., Block T., Deshmane S. L., Valyi-Nagy T., Natarajan R., Gesser R. M. A review of the molecular mechanism of HSV-1 latency. Curr Eye Res. 1991;10 (Suppl):1–13. doi: 10.3109/02713689109020352. [DOI] [PubMed] [Google Scholar]
- Gordon Y. J., Johnson B., Romanowski E., Araullo-Cruz T. RNA complementary to herpes simplex virus type 1 ICP0 gene demonstrated in neurons of human trigeminal ganglia. J Virol. 1988 May;62(5):1832–1835. doi: 10.1128/jvi.62.5.1832-1835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., McKnight J. L., Ostrove J. M., Romanowski E., Araullo-Cruz T. Host species and strain differences affect the ability of an HSV-1 ICP0 deletion mutant to establish latency and spontaneously reactivate in vivo. Virology. 1990 Oct;178(2):469–477. doi: 10.1016/0042-6822(90)90344-q. [DOI] [PubMed] [Google Scholar]
- Harbour D. A., Hill T. J., Blyth W. A. Acute and recurrent herpes simplex in several strains of mice. J Gen Virol. 1981 Jul;55(Pt 1):31–40. doi: 10.1099/0022-1317-55-1-31. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Rayfield M. A., Haruta Y. Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res. 1987 Jan;6(1):91–97. doi: 10.3109/02713688709020074. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990 Jan;174(1):117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Izumi K. M., McKelvey A. M., Devi-Rao G., Wagner E. K., Stevens J. G. Molecular and biological characterization of a type 1 herpes simplex virus (HSV-1) specifically deleted for expression of the latency-associated transcript (LAT). Microb Pathog. 1989 Aug;7(2):121–134. doi: 10.1016/0882-4010(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Javier R. T., Stevens J. G., Dissette V. B., Wagner E. K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988 Sep;166(1):254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Leib D. A., Bogard C. L., Kosz-Vnenchak M., Hicks K. A., Coen D. M., Knipe D. M., Schaffer P. A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989 Jul;63(7):2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean A. R., Brown S. M. Deletion and duplication variants around the long repeats of herpes simplex virus type 1 strain 17. J Gen Virol. 1987 Dec;68(Pt 12):3019–3031. doi: 10.1099/0022-1317-68-12-3019. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Lirette R. P., Fraser N. W. Mapping of low abundance latency-associated RNA in the trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):125–132. doi: 10.1099/0022-1317-71-1-125. [DOI] [PubMed] [Google Scholar]
- O'Brien W. J., Taylor J. L. The isolation of herpes simplex virus from rabbit corneas during latency. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):357–364. [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E., Meneguzzi G., Chenciner N., Milanesi G. Herpes simplex virus DNA isolation from infected cells with a novel procedure. Virology. 1979 Feb;93(1):260–264. doi: 10.1016/0042-6822(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y., Gangarosa L. P., Sr, Kataoka M., Hill J. M. HSV-1 shedding by lontophoresis of 6-hydroxydopamine followed by topical epinephrine. Invest Ophthalmol Vis Sci. 1983 Dec;24(12):1588–1594. [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 (HSV-1) latency-associated transcripts and transcripts affected by the deletion in avirulent mutant HFEM: evidence for a new class of HSV-1 genes. J Virol. 1988 Sep;62(9):3281–3287. doi: 10.1128/jvi.62.9.3281-3287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988 May;62(5):1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., O'Boyle D. R., 2nd, Fraser N. W. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J Virol. 1987 Oct;61(10):3288–3291. doi: 10.1128/jvi.61.10.3288-3291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., O'Boyle D. R., 2nd, Lavi E., Fraser N. W. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J Virol. 1988 Sep;62(9):3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Robin J. B., Willey D. E., De Clercq E. Intentional reactivation of latent ocular herpes infection during BVDU therapy. Curr Eye Res. 1987 Dec;6(12):1471–1477. doi: 10.3109/02713688709044511. [DOI] [PubMed] [Google Scholar]
- Willey D. E., Trousdale M. D., Nesburn A. B. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):945–950. [PubMed] [Google Scholar]
- Wroblewska Z., Spivack J. G., Otte J., Steiner I., Brown M., MacLean A., Fraser N. W. The HSV-1 latency associated transcript (LAT) variants 1704 and 1705 are glycoprotein C negative. Virus Res. 1991 Jul;20(2):193–200. doi: 10.1016/0168-1702(91)90109-9. [DOI] [PubMed] [Google Scholar]




