Abstract
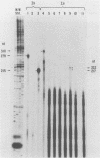
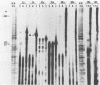
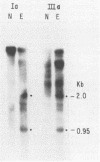
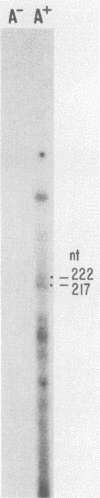
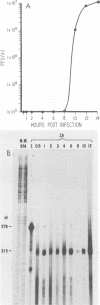
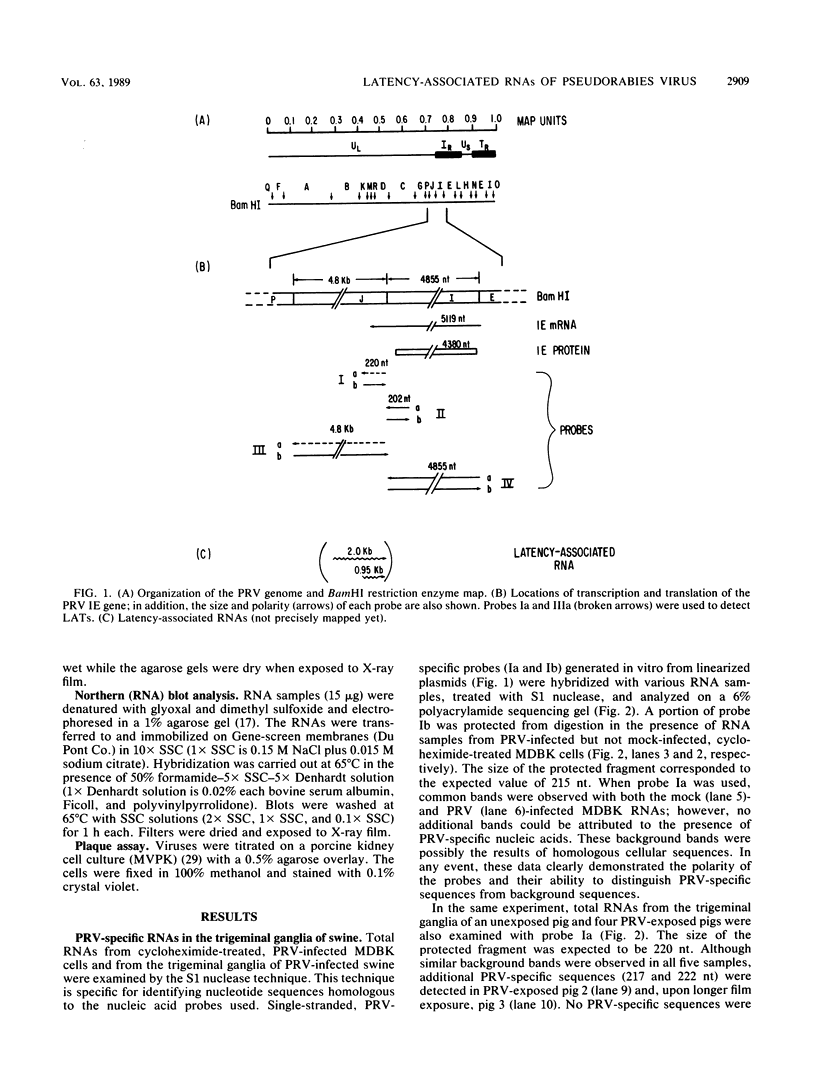
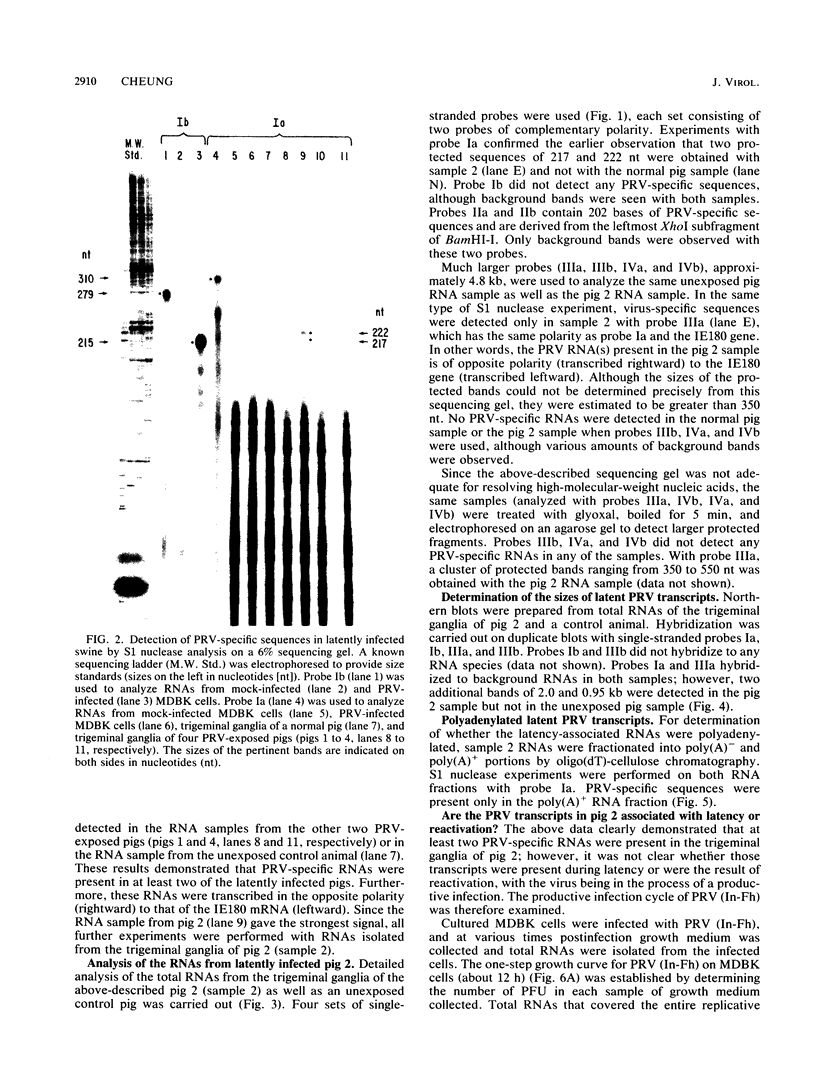
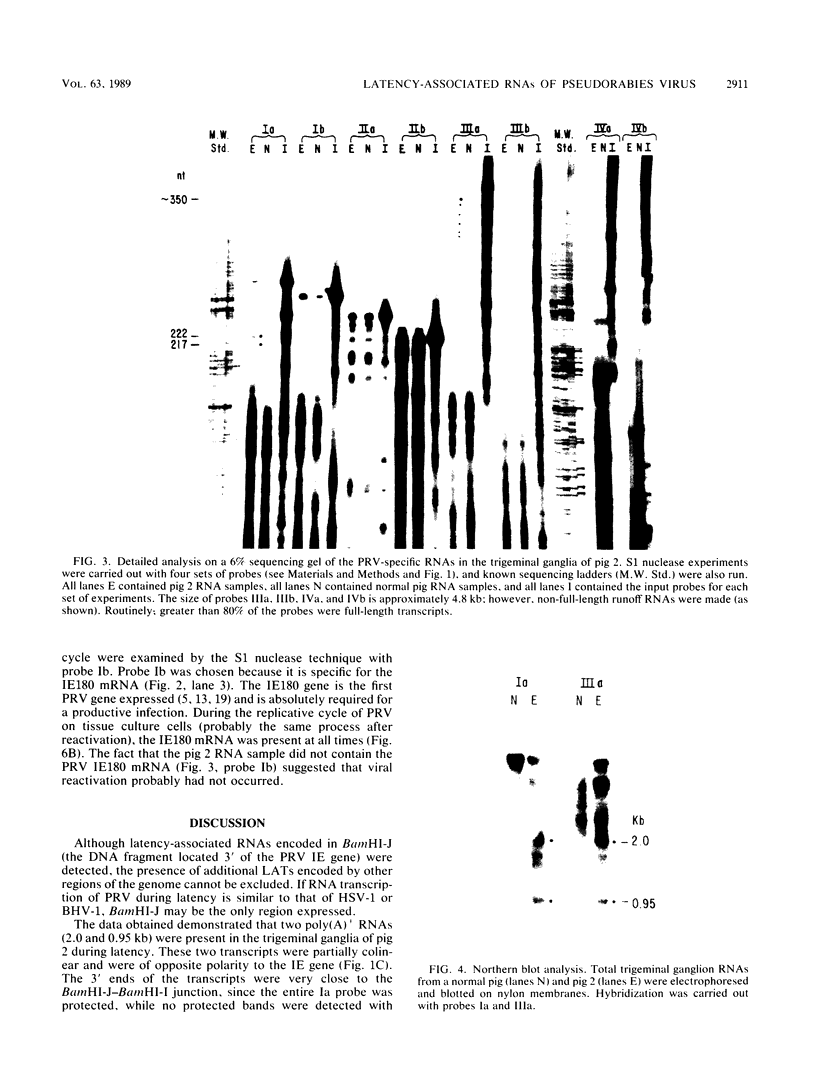
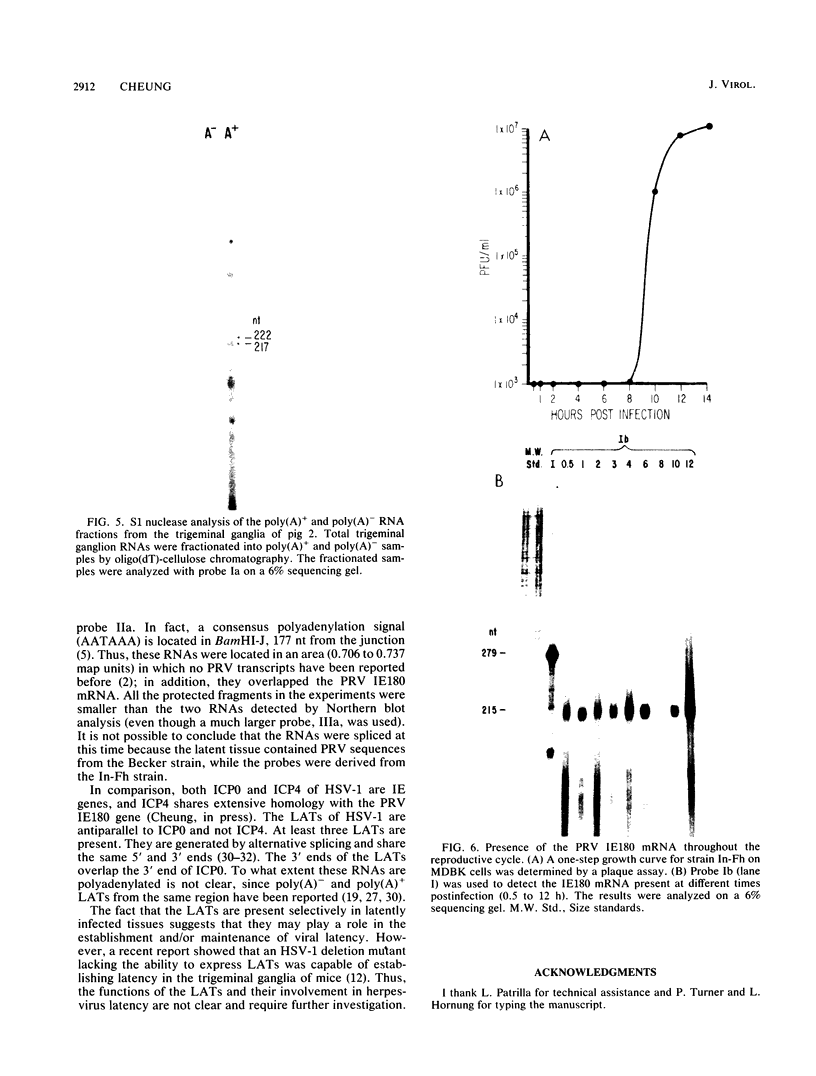
Two pseudorabies virus (PRV) poly(A)+ RNAs, 2.0 and 0.95 kilobases, were detected in the trigeminal ganglia of latently infected pigs. These RNAs were partially colinear at the 3' end. They were located in the BamHI J fragment (0.706 to 0.737 map units), 3' of the PRV immediate-early gene. A potential polyadenylation signal was also identified in BamHI-J. The direction of transcription of the latency-associated RNAs was antiparallel (i.e., opposite in polarity) to that of the PRV immediate-early mRNA.
Full text
PDF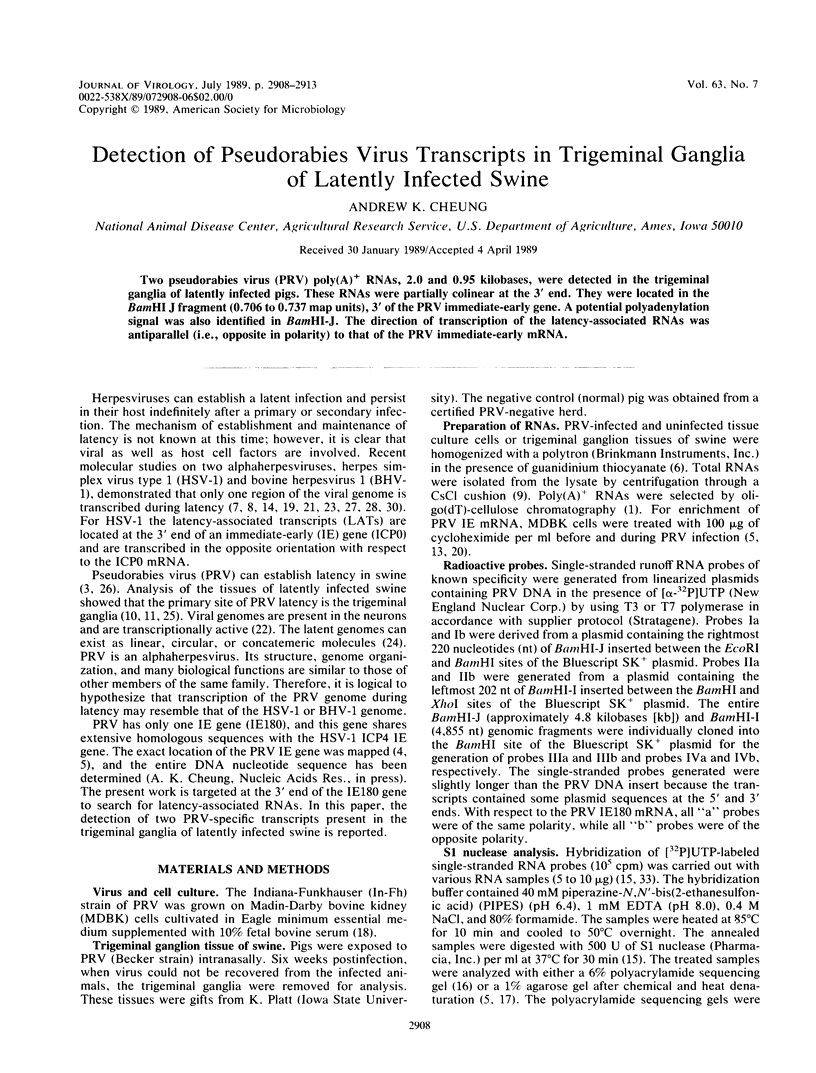

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran G. W., Davies E. B., Arambulo P. V., 3rd, Will L. A., Hill H. T., Rock D. L. Persistence of pseudorabies virus in infected swine. J Am Vet Med Assoc. 1980 May 15;176(10 Pt 1):998–1000. [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Cheung A. K. Fine mapping of the immediate-early gene of the Indiana-Funkhauser strain of pseudorabies virus. J Virol. 1988 Dec;62(12):4763–4766. doi: 10.1128/jvi.62.12.4763-4766.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gutekunst D. E. Latent pseudorabies virus infection in swine detected by RNA-DNA hybridization. Am J Vet Res. 1979 Nov;40(11):1568–1572. [PubMed] [Google Scholar]
- Gutekunst D. E., Pirtle E. C., Miller L. D., Stewart W. C. Isolation of pseudorabies virus from trigeminal ganglia of a latently infected sow. Am J Vet Res. 1980 Aug;41(8):1315–1316. [PubMed] [Google Scholar]
- Javier R. T., Stevens J. G., Dissette V. B., Wagner E. K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988 Sep;166(1):254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Jean J. H., Ben-Porat T., Kaplan A. S. Early functions of the genome of herpesvirus. 3. Inhibition of the transcription of the viral genome in cells treated with cycloheximide early during the infective process. Virology. 1974 Jun;59(2):516–523. doi: 10.1016/0042-6822(74)90461-9. [DOI] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtle E. C., Wathen M. W., Paul P. S., Mengeling W. L., Sacks J. M. Evaluation of field isolates of pseudorabies (Aujeszky's disease) virus as determined by restriction endonuclease analysis and hybridization. Am J Vet Res. 1984 Oct;45(10):1906–1912. [PubMed] [Google Scholar]
- Puga A., Notkins A. L. Continued expression of a poly(A)+ transcript of herpes simplex virus type 1 in trigeminal ganglia of latently infected mice. J Virol. 1987 May;61(5):1700–1703. doi: 10.1128/jvi.61.5.1700-1703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Hagemoser W. A., Osorio F. A., McAllister H. A. Transcription from the pseudorabies virus genome during latent infection. Brief report. Arch Virol. 1988;98(1-2):99–106. doi: 10.1007/BF01321010. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rziha H. J., Mettenleiter T. C., Ohlinger V., Wittmann G. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology. 1986 Dec;155(2):600–613. doi: 10.1016/0042-6822(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Sabó A., Grúnert Z. Persistence of virulent pseudorabies virus in herds of vaccinated and nonvaccinated pigs. Acta Virol. 1971 Jan;15(1):87–94. [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Swaney L. M. Susceptibility of a new fetal pig kidney cell line (MVPK-1) to foot-and-mouth disease virus. Am J Vet Res. 1976 Nov;37(11):1319–1322. [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol. 1988 Nov;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Zwaagstra J., Ghiasi H. Sequence of the latency-related gene of herpes simplex virus type 1. Virology. 1989 Jan;168(1):168–172. doi: 10.1016/0042-6822(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]