Abstract
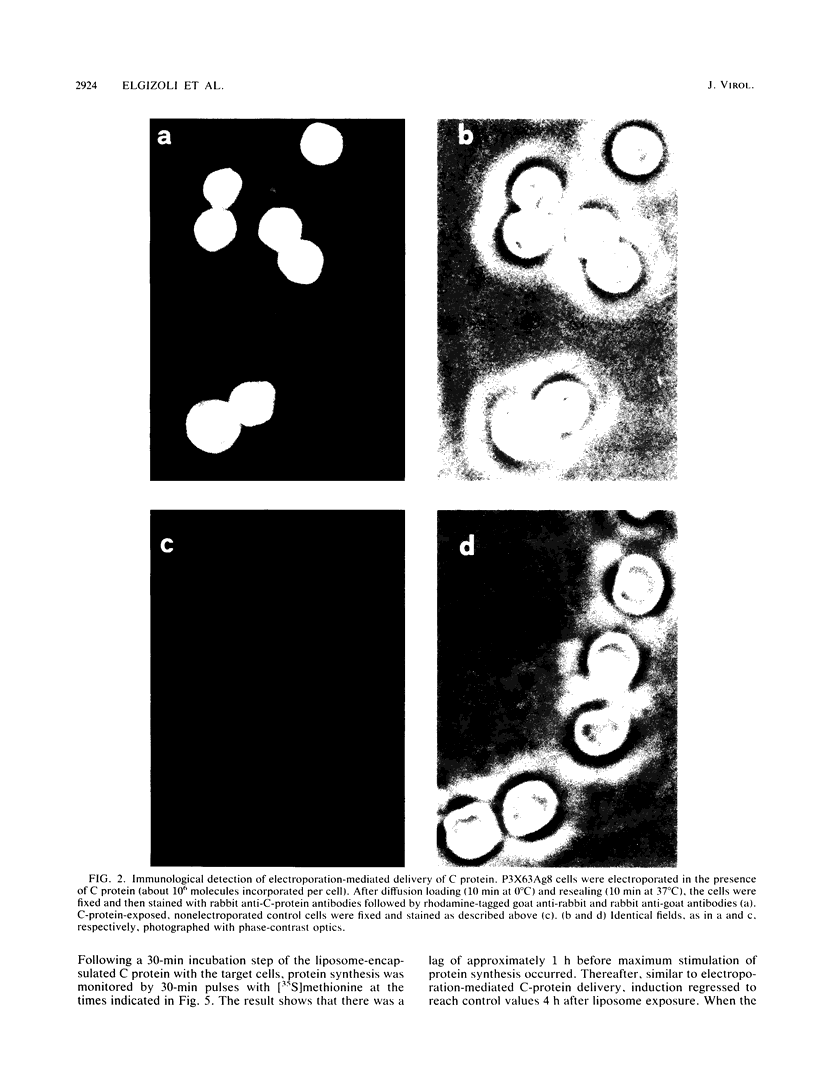
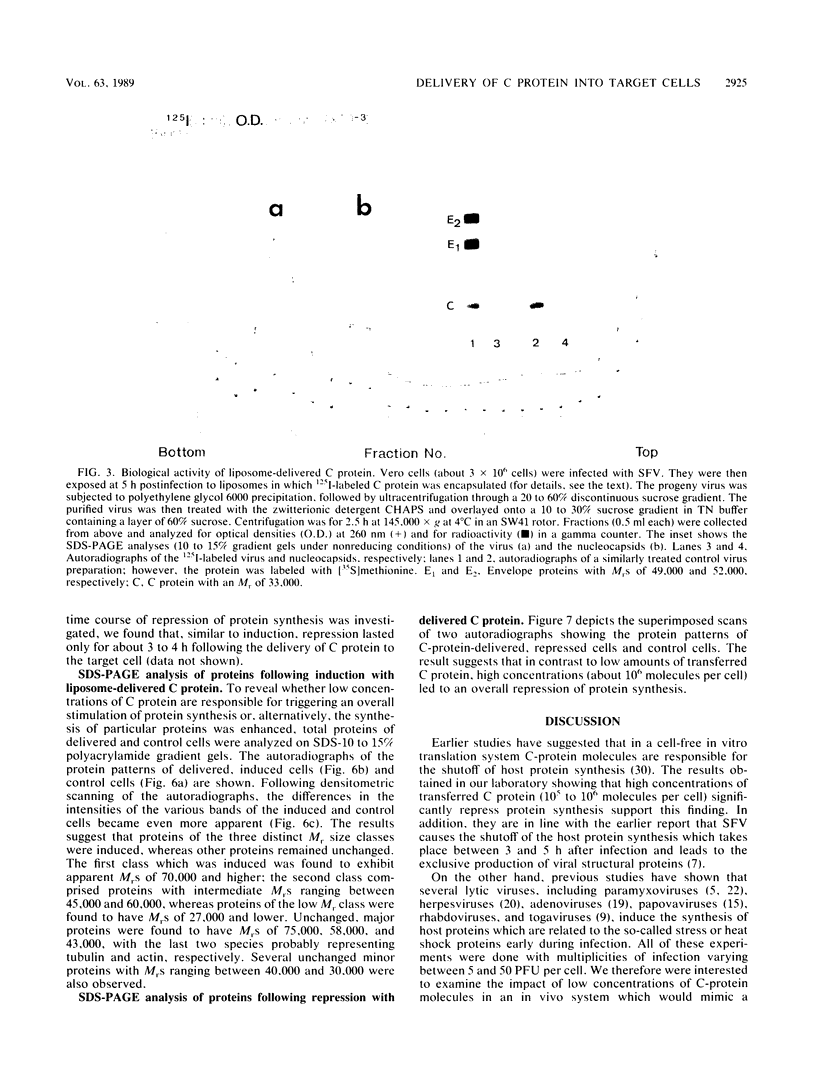
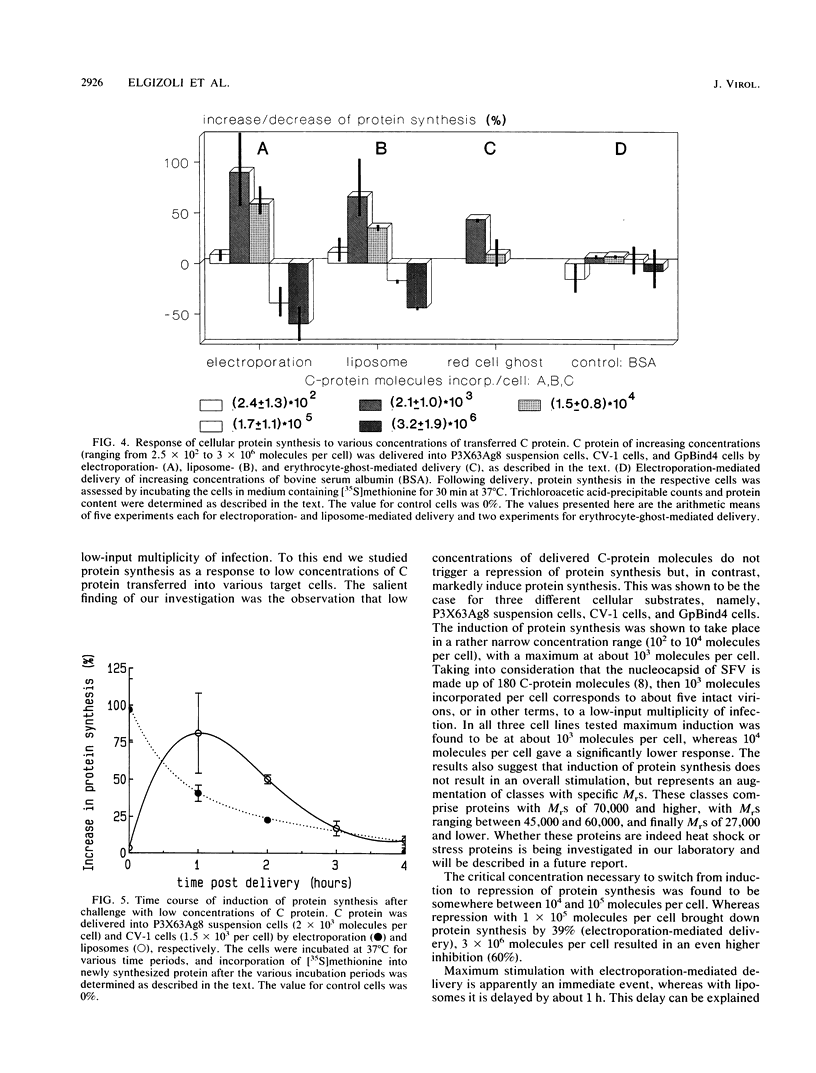
The Semliki Forest virus capsid (C) protein was introduced into various target cells by electroporation-, liposome-, and erythrocyte-ghost-mediated delivery. Data are presented which show that the incorporated C protein is biologically active and, at low concentrations (10(3) to 10(4) molecules per cell), markedly induces host cellular protein synthesis (average value, up to 90%). On the other hand, high concentrations (10(5) to 10(6) molecules per cell) led to a significant inhibition (average value, up to 60%). The cellular response to C protein was found to be identical in P3X63Ag8 suspension cells, CV-1 cells, and GpBind4 cells. Following electroporation-mediated delivery of C-protein molecules, both induction and repression of cellular protein synthesis were immediate, whereas with liposome-mediated delivery these events were delayed by about 1 h. Maximum stimulation and repression occurred between 0 and 1 h after delivery of C protein and decreased thereafter to reach control values at about 4 h. The analysis of the proteins synthesized suggests that low amounts of microinjected C protein are responsible for the induction of classes with specific Mrs, whereas high amounts lead to an inhibition of overall protein synthesis.
Full text
PDF

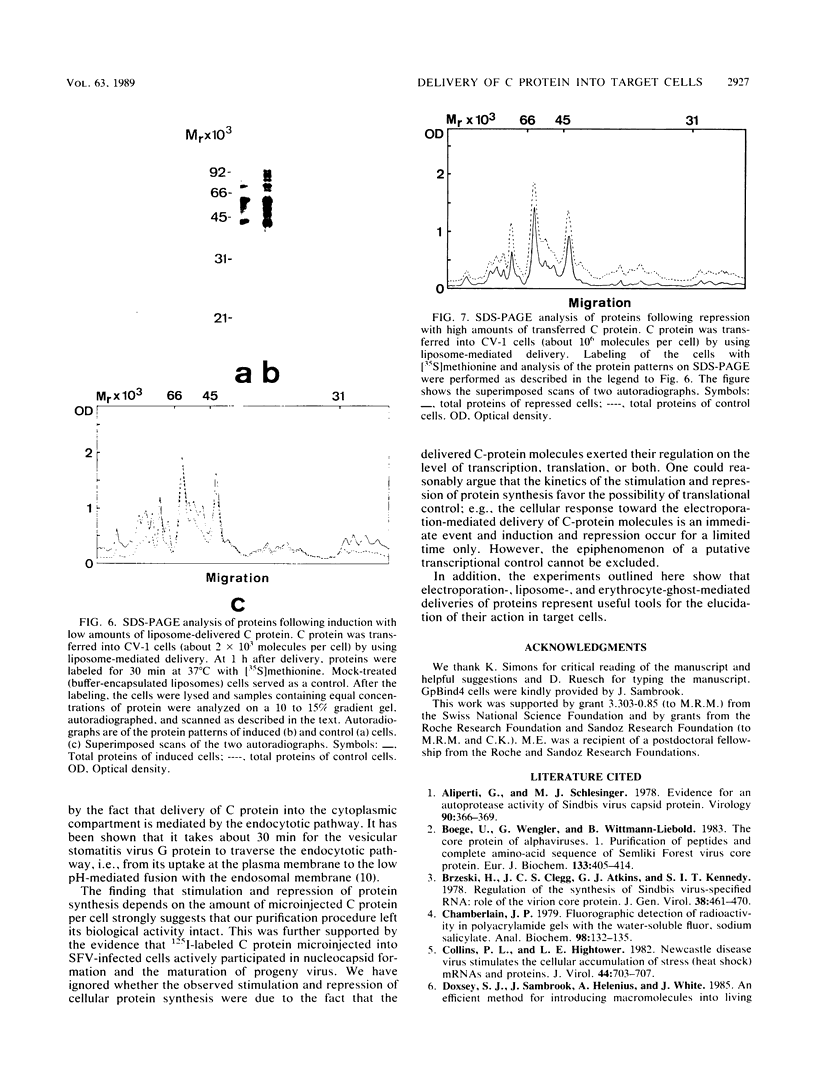

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Boege U., Wengler G., Wittmann-Liebold B. The core protein of alphaviruses. 1. Purification of peptides and complete amino-acid sequence of Semliki Forest virus core protein. Eur J Biochem. 1983 Jun 15;133(2):405–414. doi: 10.1111/j.1432-1033.1983.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Brzeski H., Clegg J. C., Atkins G. J., Kennedy S. I. Regulation of the synthesis of Sindbis virus-specified RNA: role of the virion core protein. J Gen Virol. 1978 Mar;38(3):461–470. doi: 10.1099/0022-1317-38-3-461. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Ulug E. T., Bose H. R., Jr Induction of stress proteins in Sindbis virus- and vesicular stomatitis virus-infected cells. Virology. 1983 Sep;129(2):319–332. doi: 10.1016/0042-6822(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. E., Howell K. E. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986 Dec 1;5(12):3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen L., Gomatos P. J. A kinetic analysis of the synthesis in BHK 21 cells of RNAs specific for Semliki Forest virus. J Gen Virol. 1969 Sep;5(2):251–265. doi: 10.1099/0022-1317-5-2-251. [DOI] [PubMed] [Google Scholar]
- Kaltoft K., Celis J. E. Ghost-mediated transfer of human hypoxanthine-guanine phosphoribosyl transferase into deficient Chinese hamster ovary cells by means of polyethylene glycol-induced fusion. Exp Cell Res. 1978 Sep;115(2):423–428. doi: 10.1016/0014-4827(78)90299-9. [DOI] [PubMed] [Google Scholar]
- Karonen S. L., Mörsky P., Siren M., Seuderling U. An enzymatic solid-phase method for trace iodination of proteins and peptides with 125-iodine. Anal Biochem. 1975 Jul;67(1):1–10. doi: 10.1016/0003-2697(75)90266-3. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Melancon P., Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987 May;61(5):1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Elgizoli M., Koblet H., Kempf C. Diffusion loading conditions determine recovery of protein synthesis in electroporated P3X63Ag8 cells. Experientia. 1988 Mar 15;44(3):199–203. doi: 10.1007/BF01941705. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Notarianni E. L., Preston C. M. Activation of cellular stress protein genes by herpes simplex virus temperature-sensitive mutants which overproduce immediate early polypeptides. Virology. 1982 Nov;123(1):113–122. doi: 10.1016/0042-6822(82)90299-9. [DOI] [PubMed] [Google Scholar]
- Omar A., Koblet H. One-step separation of the components of Semliki Forest virus by cation exchange chromatography. J Virol Methods. 1985 Oct;12(1-2):71–83. doi: 10.1016/0166-0934(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Rodgers L., White J., Gething M. J. Lines of BPV-transformed murine cells that constitutively express influenza virus hemagglutinin. EMBO J. 1985 Jan;4(1):91–103. doi: 10.1002/j.1460-2075.1985.tb02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger R. M., Düzgünes N., Papahadjopoulos D. pH-sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules. FEBS Lett. 1985 Jan 1;179(1):148–154. doi: 10.1016/0014-5793(85)80210-6. [DOI] [PubMed] [Google Scholar]
- Straubinger R. M., Hong K., Friend D. S., Papahadjopoulos D. Endocytosis of liposomes and intracellular fate of encapsulated molecules: encounter with a low pH compartment after internalization in coated vesicles. Cell. 1983 Apr;32(4):1069–1079. doi: 10.1016/0092-8674(83)90291-x. [DOI] [PubMed] [Google Scholar]
- Sugawa H., Uchida T., Yoneda Y., Ishiura M., Okada Y. Large macromolecules can be introduced into cultured mammalian cells using erythrocyte membrane vesicles. Exp Cell Res. 1985 Aug;159(2):410–418. doi: 10.1016/s0014-4827(85)80014-8. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Virion-bound protein kinase in Semliki forest and Sindbis viruses. J Virol. 1974 Jun;13(6):1245–1253. doi: 10.1128/jvi.13.6.1245-1253.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Role of protein synthesis in the assembly of Semliki forest virus nucleocapsid. Virology. 1979 Dec;99(2):265–276. doi: 10.1016/0042-6822(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Semliki Forest virus capsid protein associates with the 60S ribosomal subunit in infected cells. J Virol. 1976 Oct;20(1):203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G. The mode of assembly of alphavirus cores implies a mechanism for the disassembly of the cores in the early stages of infection. Brief review. Arch Virol. 1987;94(1-2):1–14. doi: 10.1007/BF01313721. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Kasperaitis M., Voorma H. O., Benne R. Infection of neuroblastoma cells by Semliki Forest virus. The interference of viral capsid protein with the binding of host messenger RNAs into initiation complexes is the cause of the shut-off of host protein synthesis. Eur J Biochem. 1984 Feb 1;138(3):473–478. doi: 10.1111/j.1432-1033.1984.tb07940.x. [DOI] [PubMed] [Google Scholar]