Abstract
Vaccines harboring genes that encode functional oncoproteins are intrinsically hazardous, as their application may lead to introduction of these genes into normal cells and thereby to tumorigenesis. On the other hand, oncoproteins are especially attractive targets for immunotherapy of cancer, as their expression is generally required for tumor growth, making the arisal of tumor variants lacking these antigens unlikely. Using murine tumor models, we investigated the efficacy of polyepitope recombinant adenovirus (rAd) vaccines, which encode only the immunogenic T cell epitopes derived from several oncogenes, for the induction of protective anti-tumor immunity. We chose to employ rAd, as these are safe vectors that do not induce the side effects associated with, for example, vaccinia virus vaccines. A single polyepitope rAd was shown to give rise to presentation of both H-2 and human leukocyte antigen-restricted cytotoxic T lymphocyte (CTL) epitopes. Moreover, vaccination with a rAd encoding H-2-restricted CTL epitopes, derived from human adenovirus type 5 early region 1 and human papilloma virus type 16-induced tumors, elicited strong tumor-reactive CTL and protected the vaccinated animals against an otherwise lethal challenge with either of these tumors. The protection induced was superior compared with that obtained by vaccination with irradiated tumor cells. Thus, vaccination with polyepitope rAd is a powerful approach for the induction of protective anti-tumor immunity that allows simultaneous immunization against multiple tumor-associated T cell epitopes, restricted by various major histocompatibility complex haplotypes.
Cytotoxic T lymphocyte (CTL)-mediated immunity plays an important role in the host defense against many tumors. However, natural CTL immunity against tumors often falls short in preventing the development of malignancies. Therefore, activation of the tumor-directed CTL response by vaccination constitutes a promising approach for the prevention and treatment of malignancies.
Induction of an effective CTL-mediated anti-tumor response against the relevant tumor-associated antigens can be achieved in various ways, including immunization with whole proteins, peptides, DNA encoding the tumor-associated antigen of choice, (genetically engineered) irradiated tumor cells, or attenuated organisms, like recombinant bacteria or viruses, expressing the tumor-associated antigens of choice (1–7). However, these vaccination approaches might not always result in the desired effect. For instance, vaccine approaches that deliver entire proteins may direct the immune response against an immunodominant T cell epitope only and not against other subdominant T cell epitopes. Alternatively, such vaccines may direct the immune response primarily against epitopes that are subjected to antigenic variation (8–11). Induction of T cell immunity against preselected T cell epitopes through peptide vaccination has in certain cases been shown to induce protective anti-tumor immunity (2, 12), but it can also lead to specific CTL tolerance induction and, thereby, to enhanced tumor outgrowth (13, 14). In the latter instances, protective CTL-mediated immunity was induced when an adenovirus vector encoding the entire tumor antigen was used for vaccination.
Together, these observations prompted us to design a vaccine that would deliver the T cell epitopes via a mode ensuring the induction of protective immunity and that is rationally designed, in the sense that only minimal essential epitopes derived from one or more tumor-associated antigens expressed by the same tumor are incorporated. The approach of using the sequences encoding immunogenic T cell epitopes derived from (onco)proteins, rather than the entire proteins, has the additional advantage of avoiding the potential hazards of immunizing with agents carrying genes that are, or may be, involved in transformation. For example, recombinant vaccines for cervical carcinoma are intrinsically hazardous if such vaccines contain the functional human papilloma virus type 16 (HPV16) early region 6 (E6) and E7 oncogenes (15, 16). The same holds true for vaccines encoding other proteins that are implicated in the development of malignancies, such as HER2/neu, the aberrant fusion protein BCR-ABL, or mutated Ras and p53 proteins. Likewise, the use of vectors encoding tumor-specific antigens of which the function has not yet been elucidated, such as the MAGE genes, has a potential risk, as the consequences of (over)expression of such genes are unknown. Through application of recombinant vectors encoding string-of-beads arrangements of oncogene-derived T cell epitopes, as opposed to vectors harboring intact oncogenes, the introduction of functional oncogenes into the patient is avoided.
Recently, recombinant vaccinia viruses expressing several virus-derived CTL epitopes in a string-of-beads fashion have been successfully used to immunize mice (17, 18). These studies show the potency of the use of string-of-beads vaccines for the induction of antiviral immunity. However, the potential risks associated with vaccinia virus, such as encephalopathy and postvaccinal encephalitis, as well as the decreasing or absent (in younger individuals) immunity to poxvirus, prohibit the large scale application of recombinant vaccinia vaccines in humans (19). High postvaccination complication rates are reported, particularly in older (those aged >4 years) primary vaccinees (20). Therefore, we studied the potential of a recombinant adenovirus vector (rAd) encoding several CTL epitopes in a string-of-beads fashion. Attractive features of adenovirus-based vaccines are their well-characterized genetic arrangement and function, as well as their extensive and safe usage in North American army recruits without inducing adverse side effects (21–23). The safety of the currently used rAd is even increased by the fact that these vectors, due to deletion of the E1 region, are largely replication defective.
Recently, promising results have been obtained by us and others using rAd encoding intact tumor-associated antigens as vaccines for the induction of protective anti-tumor immunity (13, 14, 24, 25). We now demonstrate that a rationally designed rAd vaccine, encoding a string-of-beads arrangement of several CTL epitopes derived from the human adenovirus type 5 early region 1A (Ad5E1A), E1B and HPV16 E7 oncoproteins, is a highly effective tool for the induction of protective T cell-mediated anti-tumor immunity.
MATERIALS AND METHODS
Cell Lines.
All cell lines were maintained in Iscove’s modified Dulbecco’s medium (IMDM) (Seromed, Berlin) supplemented with 4% fetal calf serum (HyClone), penicillin (110 international units/ml; Brocades Pharma, Leiderdorp, The Netherlands), and 2-mercaptoethanol (20 μM) at 37°C in a 5% CO2 atmosphere. CTL clones were cultured as described elsewhere (26–29).
Generation of rAd.
The adenoviral vector construction adapter plasmid pMad5 was derived from plasmid pMLP10 (30) as follows. pMLP10-lin was constructed by insertion of a synthetic DNA fragment with unique sites for the restriction endonucleases MluI, SplI, SnaBI, SpeI, AsuII, and MunI into the HindIII site of pMLP10. Subsequently, the adenovirus BglII fragment spanning nucleotides 3328–8914 of the Ad5 genome was inserted into the MunI site of pMLP-lin. Finally, the SalI–BamHI fragment was deleted to inactivate the tetracycline resistance gene, resulting in plasmid pMad5. A minigene cassette vector, pMad5-0, was generated by ligation of the annealed and phosphorylated double-stranded oligonucleotides 1a/b and 2a/b (see Table 1) into the MluI and SpeI sites of pMad5. This cloning step leads to elimination of the original MluI and SpeI sites and to creation of a small ORF, which essentially consists of a start codon, the sequence SEQKLISEEDLNN, a human c-Myc-derived sequence, which is recognized by mAb 9E10 (31) and a stop codon. A small “stuffer” sequence, flanked by newly generated MluI and SpeI sites, is present between the start codon and the c-Myc sequence.
Table 1.
Oligonucleoties used for the generation of rAdV
| 1a CGCGAATTATGAACGCGTC |
| 1b GTACGACGCGTTCATAATT |
| 2a GTACGCTACTAGTGAACAGAAGCTGATATCAGAGGAAGACCTAAACTGAT |
| 2b CTAGATCAGTTTAGGTCTTCCTCTGATATCAGCTTCTGTTCACTAGTAGC |
| 3a CGCGGCAGCTTCCGGTCCTTCTAACACACCTCCTGAGATAGCAGCC |
| 3b GCTATCTCAGGAGGTGTGTTAGAAGGACCGGAAGCTGC |
| 4a CTGTAAATATCAGGAATTGTTGCTACATTGCAGCTG |
| 4b CTAGCAGCTGCAATGTAGCAACAATTCCTGATATTTACAGCTGC |
| 5a CGCGGCAGCTACACTAGGAATTGTGTGCCCCATCGCAGCC |
| 5b GCGATGGGGCACACAATTCCTAGTGTAGCTGC |
| 6a GCTAGAGCCCATTACAATATTGTAACCTTTGCTGCG |
| 6b GCAAAGGTTACAATATTGTAATGGGCTCTAGCGGCT |
| 7a GCTGCCATCTACAAGAAGTCACAGCACATGGCTGCAG |
| 7b ACGCCGACGGTAGATGTTCTTCAGTGTCGTGTACCGA |
| 8a GCTGGAATCCTAGGTTTCGTCTTTACGCTAGCTGCG |
| 8b GCTAGAGTAAAGACGAAACCTAGGATTCCAGCGGCT |
| 9a GCTTATATGTTAGATTTGCAACCAGAGACAACTGCTGCAG |
| 9b AGCAGTTGTCTCTGGTTGCAAATCTAACATATAAGCCGCA |
pMad5-1 and -2, each of which harbor a multi-epitope-encoding minigene, were constructed by unidirectional cloning of the following double-stranded oligonucleotides into pMad5-0, which had been cleaved with MluI and SpeI. pMad5-1: numbers 5a/b, 6a/b, 7a/b, 4a/b; pMad5-2: numbers 3a/b, 8a/b, 9a/b, 4a/b (see Table 1). After each cloning step, the sequence of the inserts was verified by DNA sequencing. The recombinant proteins that are encoded by the resulting minigenes are depicted in Fig. 1. Expression of these minigenes is driven by the Ad5 major late promoter, which in this configuration is linked to the Ad5 immediate early enhancer, resulting in immediate early expression of the minigenes.
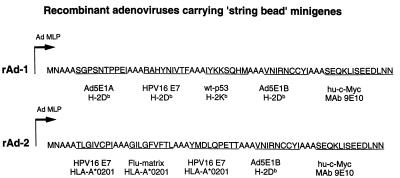
Figure 1.
Minigenes encoding several CTL epitopes, linked by a spacer of three alanines. The first minigene (rAd-1) encodes the H-2Db-restricted peptide E1A234–243 (26), the H-2Db-restricted peptide HPV16E749–57 (2), the H-2Kb-restricted peptide p53158–166 (49), the H-2Db-restricted peptide E1B192–200 (27), and a Myc tag (31). The second minigene (rAd-2) encodes the HLA-A*0201-restricted CTL epitope HPV16 E786–93 (37), the HLA-A*0201-restricted peptide Flu-matrix58–66 (48), the HLA-A*0201-restricted peptide HPV16 E711–20 (37), the H-2Db-restricted peptide E1B192–200 (27), and a Myc tag (31). Both minigenes are place behind the Ad5 major late promoter (Ad-LMP), which in this configuration is linked to the Ad5 immediate early enhancer, resulting in immediate early expression of the minigenes.
rAds were generated through in vivo homologous recombination in the Ad5E1-transformed helper cell line 911 (29) between plasmid pJM17 (32), containing the sequence of the Ad5 mutant dl309, and either of the plasmids pMad5-1 or pMad5-2. 911 cells were transfected with 10 μg of plasmid pJM17 in combination with 10 μg of either pMad5-1 or pMad5-2. The rAds were plaque-purified three times, after which the clonal rAds were propagated in 911 cells, purified by double cesium chloride density gradient centrifugation, and extensively dialyzed. The presence of replication-competent adenoviruses was routinely checked by infection of Hep-G2 cells. The viral stocks were stored in aliquots with 10% glycerol at −80°C and titered by plaque assay using 911 cells as described in ref. 29.
Transfection of COS-7 Cells.
Transient transfection in COS-7 cells was performed as described elsewhere (33). In short, 100 ng of plasmids encoding Ad5E1, HPV16 E7, murine p53, or the influenza-matrix protein together with 100 ng of a plasmid encoding H-2Db, H-2Kb, or HLA-A*0201 was transfected into 1 × 104 COS-7 cells. The transfected COS cells were incubated in 100 μl of IMDM containing 8% fetal calf serum for 48 h at 37°C, after which 1500–5000 CTL in 25 μl of IMDM containing 50 Cetus units (= 300 international units) of recombinant interleukin-2 (Cetus) were added. After 24 h, the supernatant was collected, and its tumor necrosis factor (TNF) content was determined by measuring its cytotoxic effect on WEHI-164 clone 13 cells as previously described (33).
Infection of Mouse Embryo Cells (MEC) with rAd.
C57BL/6 (B6) MEC or HLA-A2-positive SAOS cells (human osteosarcoma cells) were infected with rAd diluted in 1 ml of IMDM containing 0.5% BSA. After 30 min at room temperature, 1 ml of IMDM containing 10% fetal calf serum was added. The multiplicity of infection (moi; B6 MEC, 50; SAOS, 10) was chosen to give at least 80% infected cells. The desired moi was determined by infecting these cells with Ad.RSVβ-Gal, a rAd carrying the Escherichia coli lacZ gene under control of the promotor from the Rous sarcoma virus long terminal repeat, followed by 5-bromo-4-chloro-3-indolyl β-d-galactoside staining 48 hr later.
Generation of CTL Bulk Cultures.
Spleen cells, 5 × 106 per well, derived from B6 mice taken 2 weeks or more after intraperitoneal immunization with 1 × 108 plaque-forming units of rAd or the replication-defective adenovirus type 5 mutant Ad5ts 149, were cocultured for 6 days with 10% irradiated (25 Gy) interferon-γ (10 units/ml)-treated stimulator cells in 24-well plates. Effector cells were used in a cell-mediated lymphocyte cytotoxicity assay as previously described (27).
Peptides.
Peptides were generated by solid phase strategies on a multiple peptide synthesizer (Abimed AMS 422; Langenfeld, Germany) as described previously (34).
Tumor Cell Challenge.
B6 mice were immunized intraperitoneally with 1 × 108 plaque-forming units of rAd or the Ad5 mutant Ad5ts149 in 0.25 ml of PBS/BSA. Two weeks later, the mice were subcutaneously challenged with 0.4 × 106 Ad5E1A + Ras cells or 0.5 × 106 HPVC3 cells in 0.25 ml of PBS. Tumor volumes were measured with a caliper. Animals were sacrificed when their tumors grew larger than 1,000 mm3 to avoid unnecessary suffering.
RESULTS
The CTL Epitopes Encoded by the Constructed rAd Are Processed and Presented to Tumor-Specific CTL.
Vaccination with recombinant viruses encoding functional oncoproteins is intrinsically hazardous because it can lead to introduction of oncogenes into somatic cells and, thereby, to the development of tumor cells at the site of injection. To circumvent this problem, we constructed rAd that carried synthetic minigenes encoding a string-of-beads arrangement of minimal CTL epitopes derived from several of such oncogenes. First, minigenes were assembled in shuttle vector pMad5. Minigene 1 was designed to encode four H-2-restricted CTL epitopes that were presented by different murine tumors of B6 origin (Fig. 1), whereas minigene 2 was designed to encode the H-2Db-restricted E1B192–200 peptide (as positive control) and three HLA-A*0201-binding peptides (Fig. 1). A crucial aspect of the design of these string-of-beads minigenes is to permit proper processing of the recombinant proteins into the different immunogenic peptides. As antigenic sequences flanked by multiple alanines were shown previously to become efficiently processed relatively independent of the protein context (35), we chose to separate the CTL epitopes from each other by a spacer of three alanines.
To test whether the constructed minigenes are able to generate the CTL epitopes concerned, they were transfected into COS-7 cells together with plasmids encoding the relevant major histocompatibility complex (MHC) class I restriction elements. The transfected COS-7 cells were used in a TNF production assay as stimulator cells for different CTL clones recognizing the epitopes present in the minigenes. These experiments showed that all four epitopes encoded by minigene 1, and at least two of the four CTL epitopes, for which specific CTL clones were available (E1B192–200 and Flu58–66), encoded by minigene 2 were properly processed and presented to tumor-specific CTL (data not shown).
Subsequently, the minigenes were used to generate replication-defective rAd. H-2b-positive B6 MEC or HLA-A*0201-positive SAOS cells infected with these rAd were used as stimulator cells in a TNF production assay to analyze whether the rAd-infected cells were able to specifically activate the tumor- and virus-reactive CTL clones. Upon infection with rAd carrying minigene 1 (rAd-1), peptides E1A234–243, E1B192–200, and HPV16 E749–57 were presented by the infected cells, as the corresponding CTL were activated when incubated with B6 MEC infected with this virus but not when incubated with B6 MEC infected with a control rAd (Fig. 2). By infection of MEC derived from p53 knockout mice, we were able to show that also peptide p53158–166 was efficiently processed and presented to p53-specific CTL (data not shown). Likewise, the rAd encoding minigene 2 (rAd-2) was able to deliver peptides E1B192–200 and Flu58–66, to the surface of the infected cells in the context of H-2Db (Fig. 2) and HLA-A*0201 (Fig. 3), respectively. Processing and presentation of the HPV16 E7-derived HLA-A*0201-restricted CTL epitopes incorporated in rAd-2 could not be tested, because no CTL clones specific for these peptides are currently available. In conclusion, rAd-1 and rAd-2 are capable of delivering all preselected H-2b-restricted CTL epitopes to tumor-specific CTL, as well as the HLA-A*0201-restricted peptide Flu58–66.
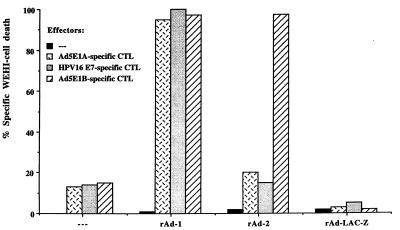
Figure 2.
CTL epitopes encoded by rAd were processed and presented to tumor-specific CTL. B6 MEC were left uninfected or were infected with rAd-1 harboring minigene 1, rAd-2, harboring minigene 2, or the galactosidase gene (rAdV-LAC-Z). After 48 hr, the infected MEC were tested for the expression of the CTL epitopes in their ability to cause TNF release by the relevant CTL. The presence of TNF in the culture supernatant was measured by the cytotoxic effect on WEHI-164 clone 13 cells. B6 MEC, infected with rAd-1 harboring E1A234–243-, HPV16 E749–57-, and E1B192–200-derived H-2Db-restricted CTL epitopes, are able to activate CTL clones specific for these CTL epitopes whereas B6 MEC infected with rAd-2 harboring the E1B192–200 epitope only activate Ad5E1B-specific CTL. The CTL are not activated upon incubation with uninfected MEC or MEC infected with a control rAd.
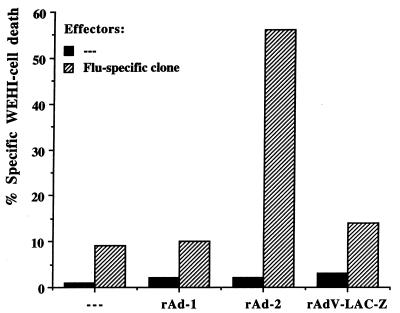
Figure 3.
The Flu-derived epitope encoded by rAd-2 is processed and presented to Flu-specific CTL. HLA-A*0201-positive SAOS cells were infected with rAd and used as stimulator cells in a TNF release assay. SAOS cells infected with rAd-2, but not with rAd-1 or rAd-LAC-Z, activate the Flu-specific CTL clone Q66.9, indicating that the Flu-derived CTL epitope is processed and presented.
Vaccination of B6 Mice with rAd Induces Tumor-Reactive CTL Activity.
Because we found rAd-1 and rAd-2 to deliver all H-2b-restricted CTL epitopes, we analyzed whether vaccination with these viruses would induce CTL activity against the epitopes concerned. Indeed, bulk T cell cultures derived from B6 mice immunized with rAd-1 showed high CTL activity against target cells loaded with the peptides E1A234–243, E1B192–200, and HPV16 E749–57 (Figs. 4 and 5). Importantly, the resulting CTL also displayed strong anti-tumor reactivity, in that tumor cells presenting the relevant CTL epitopes as endogenously processed antigen were efficiently lysed. We were not able to show p53-specific CTL reactivity under the experimental conditions used (data not shown), possibly as a result of tolerance to this self-peptide. Vaccination of B6 mice with rAd-2 induced CTL activity only against E1B192–200 and not against the E1A234–243 or HPV16 E749–57 epitopes (Fig. 5), demonstrating that immunization with rAd specifically induces CTL reactivity against the epitopes encoded by the respective minigenes. From these data, we conclude that vaccination with polyepitope rAd, which encode a recombinant polypeptide comprising several CTL epitopes in a string-of-beads fashion, induces strong tumor-specific CTL responses against the preselected epitopes.
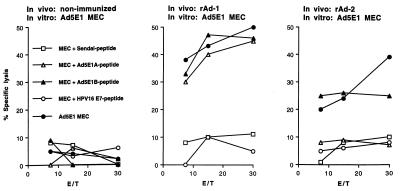
Figure 4.
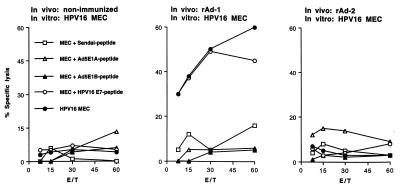
Vaccination with rAd leads to induction of tumor-reactive CTL activity against the E1-derived CTL epitopes. B6 mice were left nonimmunized, were immunized with rAd-1, harboring minigene 1, or were immunized with rAd-2, harboring minigene 2. Two weeks later, spleen cells of these animals were taken and restimulated with Ad5E1-transformed tumor cells to propagate E1A- and E1B-specific CTL. Lytic activity of bulk CTL cultures was tested 6 days later on Ad5E1 MEC, untransformed B6 MEC loaded with the Sendai-virus encoded control CTL epitope FAPGNYPAL, the E1A-encoded CTL epitope, the E1B-encoded CTL epitope, or the HPV16 E7-encoded CTL epitope. Mice immunized with rAd-1 recognize the E1A- and E1B-encoded CTL epitopes as well as tumor cells endogenously presenting these epitopes. Mice immunized with rAd-2 recognize the E1B epitope as well as tumor cells endogenously presenting this CTL epitope, whereas nonimmunized mice do not display reactivity against the target cells. Percent specific lysis at different effector to target cell ratios is shown.
Figure 5.
Vaccination with rAd leads to the induction of tumor-reactive CTL activity directed against the HPV16 E7, H-2Db-restricted CTL epitope. B6 mice were left nonimmunized, were immunized with rAd-1, harboring minigene 1, or were immunized with rAd-2, harboring minigene 2. Two weeks later, spleen cells of these animals were taken and restimulated with HPV16-transformed tumor cells to propagate H-2Db-restricted, HPV16 E7-specific CTL. Lytic activity of bulk CTL cultures was tested 6 days later on HPV16 MEC, untransformed B6 MEC loaded with the Sendai-virus encoded control CTL epitope FAPGNYPAL, the E1A-encoded CTL epitope, the E1B-encoded CTL epitope, or the HPV16 E7-encoded CTL epitope. Mice immunized with rAd-1 recognize the HPV16 E7-encoded CTL epitope as well as tumor cells endogenously presenting the HPV16 E7 epitope. Nonimmunized mice and mice immunized with rAd-2 do not display reactivity against HPV16 E7 peptide positive target cells. Percent specific lysis at different effector to target cell ratios is shown.
Induction of Protective Anti-Tumor Immunity by Vaccination with rAd.
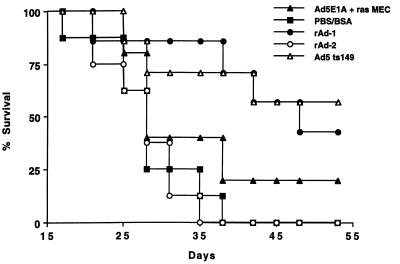
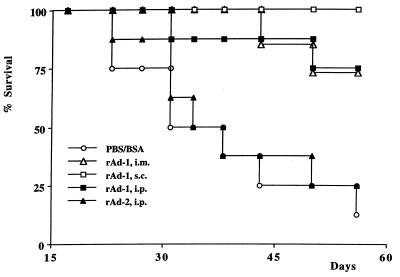
As immunization of B6 mice with rAd-1 induces both E1A- and HPV16 E7-specific CTL-mediated immunity, we studied whether this rAd-induced immunity would protect mice against a lethal challenge with tumor cells expressing the relevant oncogenes. rAd-immunized mice were challenged with either Ad5E1A + EJras- (13) or HPV16 + EJras-transformed (2) tumor cells. Indeed, mice immunized with rAd-1, in contrast to mice immunized with rAd-2 (not harboring the E1A epitope) or PBS/BSA, were protected against the outgrowth of Ad5E1A + Ras tumor cells (Fig. 6). Moreover, the protection induced by vaccination with rAd-1 was more pronounced than that obtained by vaccination with irradiated tumor cells, showing that vaccination with rAd is superior compared with vaccination with irradiated tumor cells. Mice immunized subcutaneously or intramuscularly are as equally well protected as mice immunized intraperitoneally (Fig. 7), demonstrating that rAd function as effective anti-tumor vaccines when administered via different routes.
Figure 6.
Vaccination with rAd-1 induces protective immunity against a lethal challenge with Ad5E1A-expressing tumor cells. B6 mice were immunized intraperitoneally with rAd-1 (n = 7), rAd-2 (n = 8), or the Ad5 mutant (Ad5E1A-positive) Ad5ts149 (n = 7) or were injected with PBS/BSA (n = 8) only. Two weeks later, the mice received a subcutaneous challenge of 0.4 × 106 Ad5E1A + Ras cells. Mice immunized with rAd-1 and Ad5ts149 were protected against the outgrowth of Ad5E1A + EJras cells, showing that immunization with rAd induces protective immunity against tumors.
Figure 7.
Different routes of vaccination with rAd induce protective anti-tumor immunity. B6 mice were immunized intraperitoneally (i.p.) (n = 8), intramuscularly (i.m.) (n = 8), or subcutaneously (s.c.) (n = 8) with rAd-1, immunized with PBS/BSA (n = 8), or immunized i.p. with rAd-2 (n = 8). Two weeks later, the mice received a subcutaneous challenge of 0.4 × 106 Ad5E1A + Ras cells. Mice immunized intraperitoneally, intramuscularly, or subcutaneously with rAd-1 are protected against Ad5E1A + EJras cells.
rAd-1-immunized mice were protected not only against Ad5E1A + Ras tumors but also against an otherwise lethal challenge with HPV16 + Ras-transformed tumor cells (Table 2). Thus, vaccination with polyepitope rAd constitutes a powerful method to induce protective anti-tumor immunity directed against multiple selected CTL epitopes derived from different oncogene products.
Table 2.
Vaccination with rAd-1 induces protective immunity against HPV16-transformed tumors
| Treatment | Tumor-take* |
|---|---|
| Exp. 1 | |
| None | 8/10 |
| rAd-1 | 2/10 |
| Ad5ts149 | 6/10 |
| Exp. 2 | |
| None | 10/11 |
| rAd-1 | 3/10 |
| Ad5ts149 | 10/10 |
B6 mice were left untreated or were immunized intraperitoneally with 1 × 108 plaque-forming units of rAd-1 or Ad5ts 149 as control. Two weeks later, the mice received a subcutaneous challenge of 0.5 × 106 HPV16-transformed tumor cells (2). (8/10 vs. 2/10, P < 0.05; 10/11 vs. 3/10, P < 0.01; Tukey–Kramer multiple comparisons test.)
Tumor-take is depicted at 3 weeks after tumor cell challenge as tumor-bearing animals/number of mice.
DISCUSSION
A single vaccination with rAd encoding multiple CTL epitopes, derived from different oncoproteins and arranged in a string-of-beads fashion, can elicit CTL responses against these epitopes. The resulting CTL are capable of lysing tumor cells presenting these peptides. The ability to induce CTL responses against oncoprotein-derived peptides by immunization with such polyepitope rAd offers a clear advantage over immunization strategies in which vectors carrying functional oncogenes are used, in that it largely eliminates the risk of transformation of recombinant vector-infected cells. Moreover, CTL activity can be induced against multiple (onco)gene products expressed by the same tumor cell, as illustrated by the induction of both E1A- and E1B-specific immunity after immunization with rAd-1. The similar approach can be taken for cervical carcinoma. In the majority of cervical carcinomas, both the HPV-encoded oncoproteins E6 and E7 are constitutively expressed because they are required for maintenance of the transformed state (15, 16). By introducing into rAd the HPV16 E6 and E7-derived CTL epitopes that bind to HLA-A1, A2, A3, A11, and A24 (36, 37), a powerful and versatile polyepitope vaccine against HPV16-induced cervical carcinoma may be created. As this vaccine would induce CTL immunity against multiple epitopes derived from two proteins required by the tumor for malignant growth, the risk of tumor-immune escape by antigen loss or antigen mutation is relatively small. Furthermore, based on the occurrence of the different HLA-A alleles in the caucasian population, this vaccine could be applied to the majority (>90%) of people in this population.
Many of the CTL epitope-specific vaccines directed against oncogenic proteins, which have been tested in previous studies, were based on synthetic peptides. Although in many cases peptide-based anti-tumor vaccination strategies were successful (2, 12), in other cases such vaccines were shown to induce CTL unresponsiveness, leading to enhanced tumor outgrowth (13, 14). The latter was shown by immunization with synthetic peptides representing the CTL epitopes E1A234–243 or E1B192–200, the same peptides that are incorporated in our rAd vaccines. Vaccination with these peptides induced a functional deletion of E1A- and E1B-specific CTL, respectively, and led to enhanced outgrowth of Ad5E1(A)-transformed tumor cells. We now show that these CTL epitopes, when delivered by rAd, induce protective anti-tumor immunity, demonstrating the advantage of rAd over synthetic peptides for use in epitope-specific vaccination.
Two of the four CTL epitopes present in rAd-1, E1A234–243 and HPV16 E749–57, induce only suboptimal CTL responses when irradiated tumor cell are used for vaccination (5, 14, 28). The CTL response induced by immunization with HPV16-induced tumor cells is predominantly directed against a hitherto unidentified CTL epitope (28), whereas immunization with Ad5E1-transformed tumor cells, expressing both the E1A- and E1B-encoded CTL epitopes, is primarily directed against peptide E1B192–200 (5, 14). The “subdominant” nature of the E1A epitope, when present in a tumor cell vaccine, is also reflected by the fact that vaccination with irradiated Ad5E1A-expressing tumor cells induces only a marginal protection against a challenge with Ad5E1A + Ras cells. In contrast, vaccination with rAd-1 elicits strong “co-dominant” CTL responses directed against all three CTL epitopes.
For optimal CTL induction by vaccines that encode several CTL epitopes in a string-of-beads fashion, it is obligatory that all CTL epitopes are properly processed and presented in the context of MHC class I glycoproteins. In the MHC class I pathway of antigen presentation, cellular proteins are degraded in the cytosol, resulting in peptides that are transported by TAP into the endoplasmatic reticulum (ER). Here, they assemble with the MHC class I heavy chain and β2-microglobulin to the trimolecular MHC complex (38). Consequently, the efficiency by which proteolytic degradation in the cytosol and transportation of the resulting peptides into the ER takes place is a key factor in determining whether a given peptide is presented in the context of MHC class I molecules. Indeed, B6 MEC derived from transporter associated with antigen processing (TAP)−/− mice, when infected with rAd-1 or rAd-2, were not able to stimulate the E1A-, HPV16 E7-, p53-, or E1B-specific CTL (data not shown), indicating that TAP-mediated transport of the rAd-encoded CTL epitopes into the ER is mandatory. One of the major proteolytic systems responsible for degradation of cytosolic proteins is the proteasome complex (39). It has been shown that the cleavage preference of the proteasome can define the antigenic potential of a protein (40) or even result in a lack of antigen presentation of CTL epitopes (41). Our data indicate that in polyepitope rAd-infected cells, the generation and subsequent transportation of the CTL epitopes was efficient, ensuring CTL activation in vitro and in vivo. Nonetheless, flanking the CTL epitopes with proteasomal cleavage sequences other than the triple alanine used in our minigene constructs might further improve the ability of polyepitope rAd to elicit immune responses (42).
The ability to produce large quantities of purified virus with relative ease makes rAd very attractive for clinical use. Most importantly, rAd can be safely applied to human beings because they rarely integrate into the host genome and therefore have little chance to activate a dormant oncogene or disable a tumor suppressor gene (43). Adenoviruses are extensively and safely used in vaccination of North American army recruits without causing side effects (21, 22). Furthermore, the use of rAd vectors from which the E1 region is deleted minimizes the risk of propagation of the virus, as replication of such viruses under physiological conditions is severely compromised. Recently, rAd have been applied both in nonhuman primates and in phase I clinical trials for the correction of the genetic deficiency underlying cystic fibroses without inducing any severe side effects or toxicity (44–47). Using well defined murine tumor models, we now demonstrate that polyepitope rAd constitute powerful vaccines that can be employed for the induction of strong tumor-protective CTL-mediated immunity directed against different transforming oncogene products. Tumor protection was not only induced by intraperitoneal injection of rAd but also after vaccination routes that are easy to carry out in patients (subcutaneous and intramuscular). Polyepitope rAd, therefore, have significant potential for use as vaccines in the immunotherapy of cancer, as well as in infectious diseases such as AIDS, which requires multiple T cell responses to be activated and boosted.
Acknowledgments
This work was supported by the Dutch Cancer Foundation (Grants 93-588, 95-1089, and 97-1450) and The Netherlands Organization for Scientific Research (Program PGN 901-01-096).
ABBREVIATIONS
- Ad5E1A
adenovirus type 5 early region 1A
- B6
C57BL/6
- CTL
cytotoxic T lymphocyte
- ER
endoplasmic reticulum
- HLA
human leukocyte antigen
- HPV 16
human papilloma virus type 16
- MEC
mouse embryo cells
- MHC
major histocompatibility complex
- moi
multiplicity of infection
- rAd
recombinant adenovirus(es)
- TNF
tumor necrosis factor
References
- 1.Falo L D, Kovacsovics-Bankowski M, Thompson K, Rock K L. Nat Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 2.Feltkamp M C W, Smits H L, Vierboom M P M, Minnaar R P, De Jongh B M, Drijfhout J W, Ter Schegget J, Melief C J M, Kast W M. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 3.Zerrahn J, Utermöhlen O, Warnecke G, Deppert W, Lehmann-Grube F. J Immunol. 1996;156:3919–3924. [PubMed] [Google Scholar]
- 4.Bright R K, Beames B, Shearer M H, Kennedy R C. Cancer Res. 1996;56:1126–1130. [PubMed] [Google Scholar]
- 5.Toes R E M, Blom R J J, van der Voort E, Offringa R, Melief C J M, Kast W M. Cancer Res. 1996;56:3782–3787. [PubMed] [Google Scholar]
- 6.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Bronte V, Chen P W, Gritz L, Panicali D, Rosenberg S A, Restifo N P. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 8.Sercarz E E, Lehmann P L, Ametani A, Benichou G, Miller A, Moudgil K. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 9.Maryanski J L, Boon T. Eur J Immunol. 1982;12:401–406. doi: 10.1002/eji.1830120508. [DOI] [PubMed] [Google Scholar]
- 10.Lill N L, Tevethia J, Hendrickson W G, Tevethia S S. J Exp Med. 1992;176:449–457. doi: 10.1084/jem.176.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Most R G, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau L L, Southwood S, Sidney J, Chesnut R W, Matloubain M, Ahmed R. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 12.Minev B R, McFarland B J, Spiess P J, Rosenberg S A, Restifo N P. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 13.Toes R E M, Offringa R, Blom R J J, Melief C J M, Kast W M. Proc Natl Acad Sci USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toes R E M, Blom R J J, Offringa R, Kast W M, Melief C J M. J Immunol. 1996;156:3911–3918. [PubMed] [Google Scholar]
- 15.Seedorf K, Oltersdorf T, Krämmer G, Röwekamp W. EMBO J. 1987;6:139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Knebel Doeberitz M, Bauknecht T, Bartsch D, Zur Hausen H. Proc Natl Acad Sci USA. 1991;88:1411–1415. doi: 10.1073/pnas.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitton J L, Sheng N, Oldstone M B A, McKee T A. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson S A, Elliott S L, Sherritt M A, Sproat K W, Coupar B E H, Scalzo A A, Forbes C A, Ladhams A M, Mo X Y, Tripp R A, Doherty P C, Moss D J, Suhrbier A. J Immunol. 1996;157:822–826. [PubMed] [Google Scholar]
- 19.Behbehani A M. Microbiol Rev. 1983;47:455–599. doi: 10.1128/mr.47.4.455-509.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurvich E B. Vaccine. 1992;10:96–97. doi: 10.1016/0264-410x(92)90023-d. [DOI] [PubMed] [Google Scholar]
- 21.Couch R B, Chanock R M, Cate T R, Lang D J, Knight V, Huebner R J. Am Rev Respir Dis. 1963;88:394–403. doi: 10.1164/arrd.1963.88.3P2.394. [DOI] [PubMed] [Google Scholar]
- 22.Top F H, Buescher E L, Bancroft W H, Russell P K. J Infect Dis. 1971;124:155–160. doi: 10.1093/infdis/124.2.155. [DOI] [PubMed] [Google Scholar]
- 23.Imler J-L. Vaccine. 1995;13:1143–1151. doi: 10.1016/0264-410x(95)00032-v. [DOI] [PubMed] [Google Scholar]
- 24.Chen P W, Wang M, Bronte V, Zhai Y, Rosenberg S A, Restifo N P. J Immunol. 1996;156:224–231. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai Y, Yang J C, Kawakami Y, Spiess P, Wadsworth S C, Cardoza L M, Couture L A, Smith A E, Rosenberg S A. J Immunol. 1996;156:700–710. [PubMed] [Google Scholar]
- 26.Kast W M, Offringa R, Peters P J, Voordouw A C, Meloen R H, Van der Eb A J, Melief C J M. Cell. 1989;59:603–615. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 27.Toes R E M, Offringa R, Blom H J J, Brandt R M P, Van der Eb A J, Melief C J M, Kast W M. J Immunol. 1995;154:3396–3405. [PubMed] [Google Scholar]
- 28.Feltkamp M C W, Vreugdenhil G R, Vierboom M P M, Ras E, Van der Burg S H, Ter Schegget J, Melief C J M, Kast W M. Eur J Immunol. 1995;25:2638–2641. doi: 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 29.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, Van Ormondt H, Hoeben R C, Van der Eb A J. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 30.Levrero M, Barban V, Manteca S, Ballay A, Balsamo C, Avantaggiati M L, Natoli G, Skellekens H, Tiollais P, Perricaudet M. Gene. 1991;101:195–202. doi: 10.1016/0378-1119(91)90411-4. [DOI] [PubMed] [Google Scholar]
- 31.Evan G I, Lewis G K, Ramsay G, Bishop M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrory J, Bautista D, Graham F L. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 33.Traversari C, Van der bruggen P, Van den Eynde B, Hainaut P, Lemoine C, Ohta N, Old L, Boon T. Immunogenetics. 1992;35:145–152. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- 34.Gausepohl H, Kraft M, Boulin C, Frank R W. In: 11th American Peptide Symposium. Rivier J E, Marshall G R, editors. Leiden, The Netherlands: ESCOM; 1990. pp. 1003–1004. [Google Scholar]
- 35.Del Val M, Schlicht H-J, Ruppert T, Reddehase M J, Koszinowski U. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 36.Kast W M, Brandt R M P, Sidney J, Drijfhout J W, Kubo R T, Grey H M, Melief C J M, Sette A. J Immunol. 1994;152:3904–3912. [PubMed] [Google Scholar]
- 37.Ressing M E, Sette A, Brandt R M P, Ruppert J, Wentworth P A, Hartman M, Oseroff C, Grey H M, Melief C J M, Kast W M. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 38.Neefjes J J, Momburg F. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Navarette V, Seelig A, Gernold M, Frentzel S, Kloetzel P-M, Hämmerling G J. Nature (London) 1991;353:662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- 40.Eggers M, Boes-Fabian B, Ruppert T, Kloetzel P-M, Koszinowski U. J Exp Med. 1995;182:1865–1870. doi: 10.1084/jem.182.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, Mengedé E, Kloetzel P-M, Neefjes J, Koszinowski U, Melief C J M. Immunity. 1996;5:115–124. doi: 10.1016/s1074-7613(00)80488-4. [DOI] [PubMed] [Google Scholar]
- 42.Niedermann G, Butz S, Ihlenfeldt H G, Grimm R, Lucchiari M, Hoschützky H, Jung G, Maier B, Eichmann K. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 43.Kremer E J, Perricaudet M. Br Med Bull. 1995;51:31–44. doi: 10.1093/oxfordjournals.bmb.a072951. [DOI] [PubMed] [Google Scholar]
- 44.Goldman M J, Litzky L A, Engelhardt J F, Wilson J M. Hum Gene Ther. 1995;6:839–851. doi: 10.1089/hum.1995.6.7-839. [DOI] [PubMed] [Google Scholar]
- 45.Sené C, Bout A, Inmler J-L, Schultz H, Willemot J-M, Hennebel V, Zurcher C, Valerio D, Lamy D, Pavirani A. Hum Gene Ther. 1995;6:1587–1593. doi: 10.1089/hum.1995.6.12-1587. [DOI] [PubMed] [Google Scholar]
- 46.Zabner J, Couture L A, Gregory R J, Graham S M, Smith A E, Welsh M J. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 47.Boucher R C, Knowles M R. Hum Gen Ther. 1994;5:615–639. doi: 10.1089/hum.1994.5.5-615. [DOI] [PubMed] [Google Scholar]
- 48.Bednarek M A, Sauma S Y, Gammon M C, Porter G, Tamhankar S, Williamson A R, Zweerink H J. J Immunol. 1991;147:4047–4053. [PubMed] [Google Scholar]
- 49.Vierboom M P M, Nijman H W, Offringa R, Van Der Voort E I H, van Hall T, van den Broek L, Fleuren G J, Kenemans P, Kast W M, Melief C J M. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]