Abstract
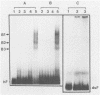
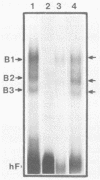
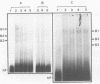
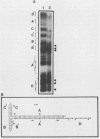
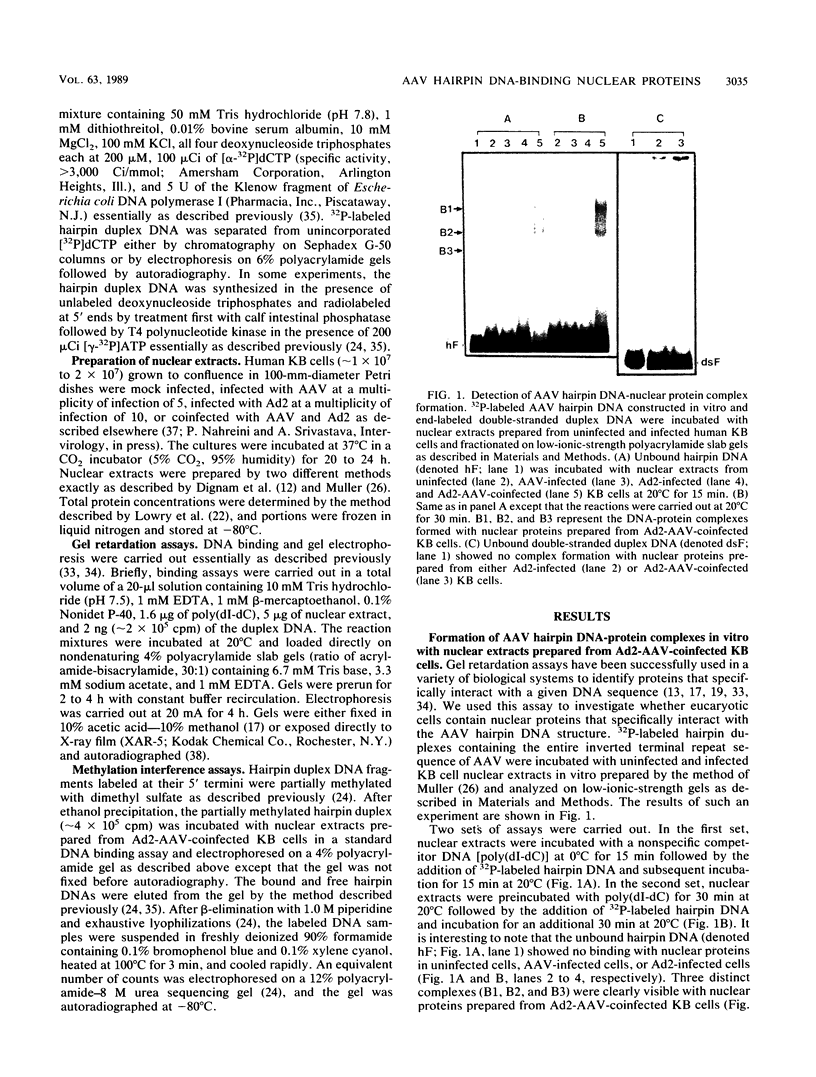
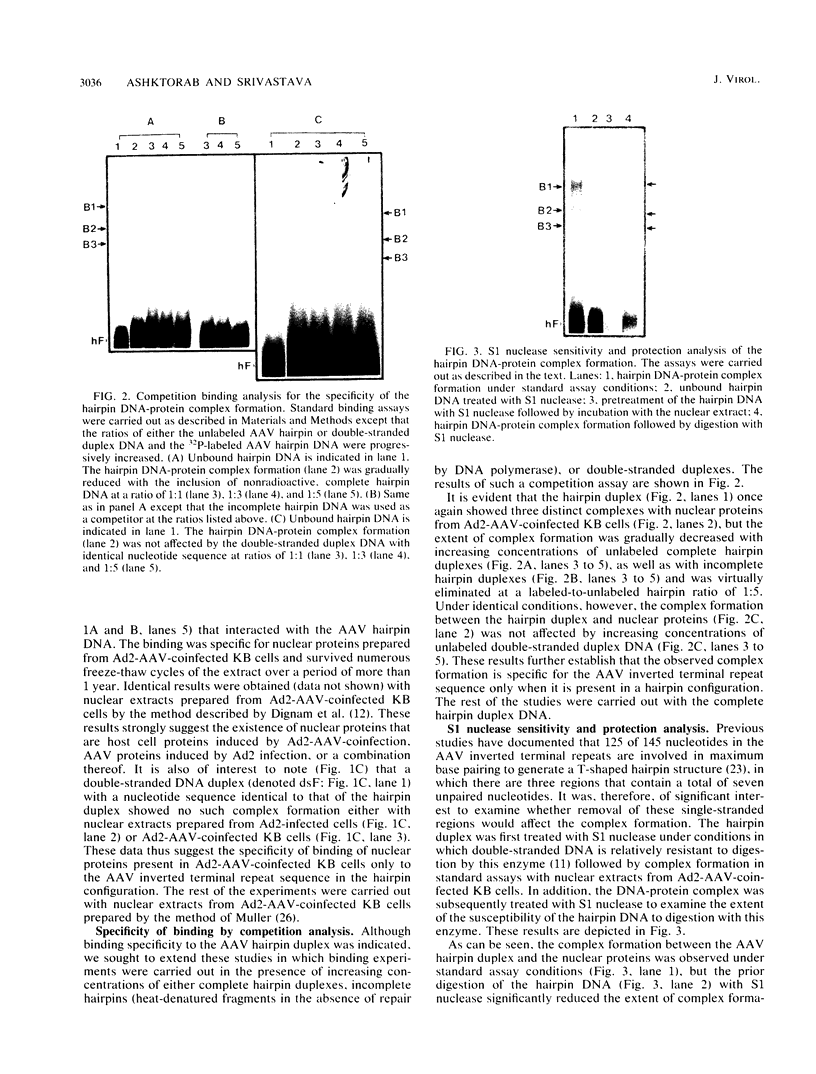
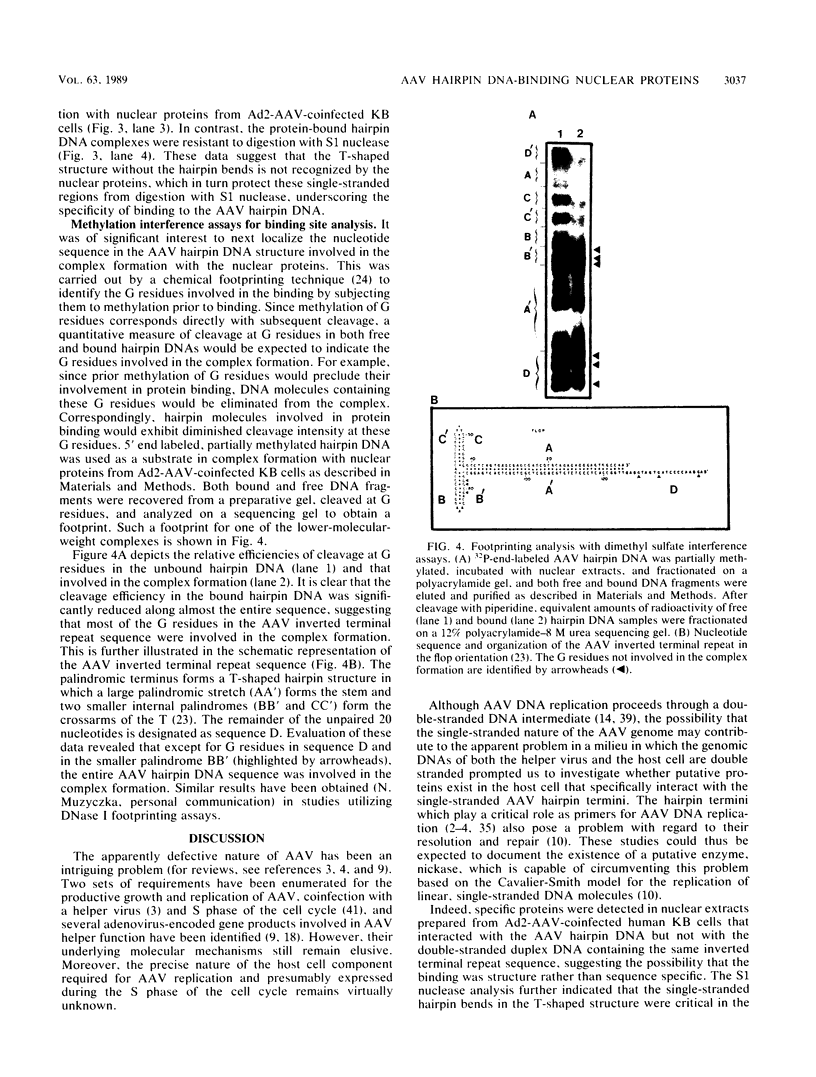
A palindromic hairpin duplex containing the inverted terminal repeat sequence of adeno-associated virus type 2 (AAV) DNA was used as a substrate in gel retardation assays to detect putative proteins that specifically interact with the AAV hairpin DNA structures. Nuclear proteins were detected in extracts prepared from human KB cells coinfected with AAV and adenovirus type 2 that interacted with the hairpin duplex but not in nuclear extracts prepared from uninfected, AAV-infected, or adenovirus type 2-infected KB cells. The binding was specific for the hairpin duplex, since no binding occurred with a double-stranded DNA duplex with the identical nucleotide sequence. Furthermore, in competition experiments, the binding could be reduced with increasing concentrations of the hairpin duplex but not with the double-stranded duplex DNA with the identical nucleotide sequence. S1 nuclease assays revealed that the binding was sensitive to digestion with the enzyme, whereas the protein-bound hairpin duplex was resistant to digestion with S1 nuclease. The nucleotide sequence involved in the protein binding was localized within the inverted terminal repeat of the AAV genome by methylation interference assays. These nuclear proteins may be likely candidates for the pivotal enzyme nickase required for replication or resolution (or both) of single-stranded palindromic hairpin termini of the AAV genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohenzky R. A., LeFebvre R. B., Berns K. I. Sequence and symmetry requirements within the internal palindromic sequences of the adeno-associated virus terminal repeat. Virology. 1988 Oct;166(2):316–327. doi: 10.1016/0042-6822(88)90502-8. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Janik J. E., Sebring E. D., Rose J. A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981 Oct;40(1):241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Dayton M. A., Nahreini P., Srivastava A. Augmented nuclease activity during cellular senescence in vitro. J Cell Biochem. 1989 Jan;39(1):75–85. doi: 10.1002/jcb.240390109. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Multiple protein-binding sites in an intracisternal A particle long terminal repeat. J Virol. 1988 Nov;62(11):4070–4077. doi: 10.1128/jvi.62.11.4070-4077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Adeno-associated virus DNA replication: nonunit-length molecules. Virology. 1979 Feb;93(1):57–68. doi: 10.1016/0042-6822(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Devaux B., Kédinger C. The abundance and in vitro DNA binding of three cellular proteins interacting with the adenovirus EIIa early promoter are not modified by the EIa gene products. Mol Cell Biol. 1987 Oct;7(10):3806–3817. doi: 10.1128/mcb.7.10.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen S., Banerjee R., Zelent A., Price P., Acs G. Identification of protein-binding sites in the hepatitis B virus enhancer and core promoter domains. Mol Cell Biol. 1988 Dec;8(12):5159–5165. doi: 10.1128/mcb.8.12.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby E., Fife K. H., Berns K. I. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980 May;34(2):402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mendelson E., Trempe J. P., Carter B. J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986 Dec;60(3):823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Berns K. I., Tan M., Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987 Oct;61(10):3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol. 1988 Aug;62(8):3059–3063. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Norris J. S., Shmookler Reis R. J., Goldstein S. c-Ha-ras-1 proto-oncogene amplification and overexpression during the limited replicative life span of normal human fibroblasts. J Biol Chem. 1985 May 25;260(10):6404–6409. [PubMed] [Google Scholar]
- Srivastava A. Replication of the adeno-associated virus DNA termini in vitro. Intervirology. 1987;27(3):138–147. doi: 10.1159/000149732. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]