Abstract
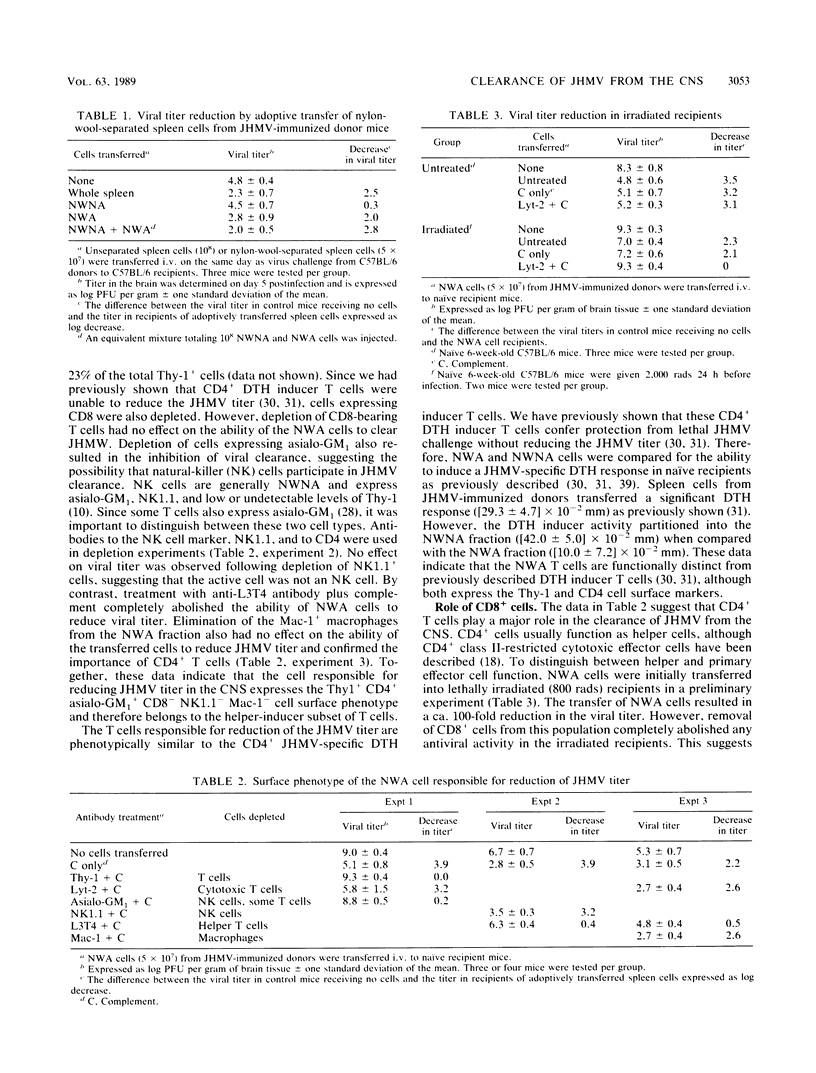
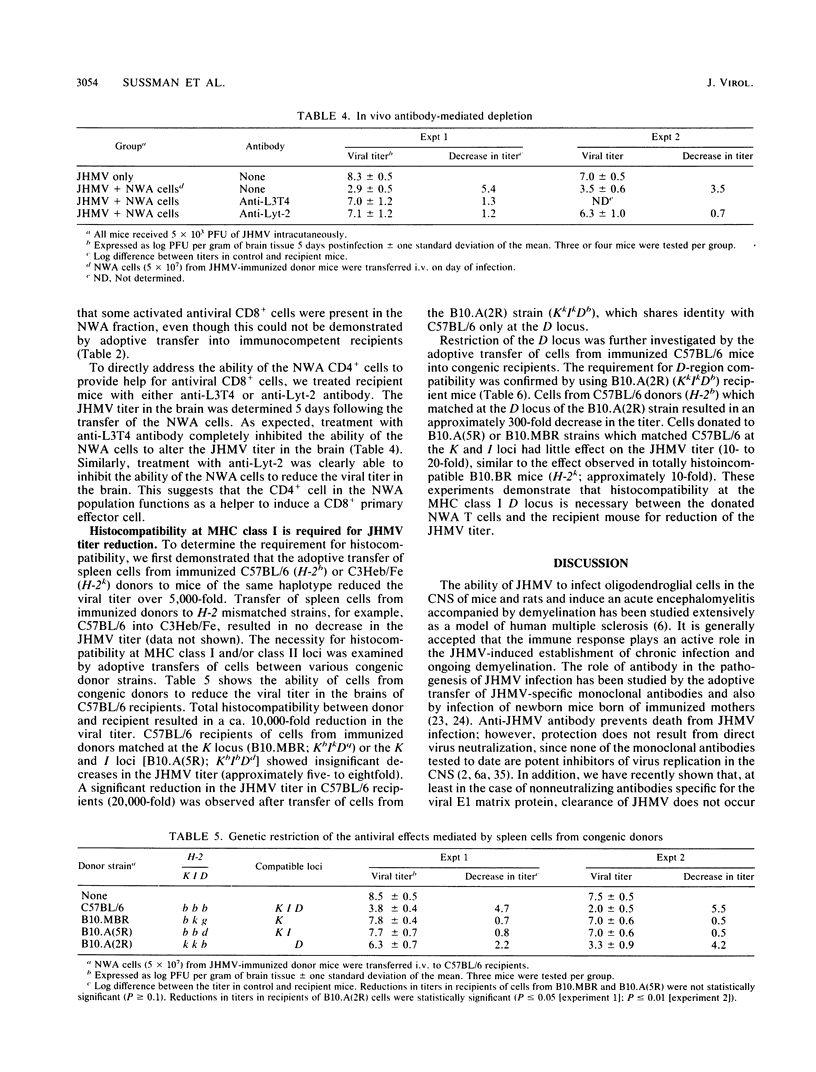
Clearance of the neurotropic JHM strain of mouse hepatitis virus from the central nervous system was examined by the transfer of spleen cells from immunized donors. A T cell with the surface phenotype of Thy1.2+ CD4+ CD8- asialo-GM1+ Mac-1- was found to be necessary for viral clearance. The surface phenotype and adherence to nylon wool suggest that these cells are activated helper-inducer T cells. Adoptive transfer to congenic histocompatibility strains demonstrated the necessity for compatibility at the D locus of the major histocompatibility complex. The expression of the CD4 surface marker and the requirement for major histocompatibility complex class I were further studied by the transfer of cells to recipients treated with anti-CD4 or anti-CD8 monoclonal antibodies. Treatment of recipients with either the anti-CD8 or the anti-CD4 antibodies inhibited virus clearance from the central nervous system. This suggests that the CD4+ cell acts as a helper and that virus is cleared from the central nervous system. This suggests that the CD4+ cell acts as a helper and that virus is cleared from the central nervous system by CD8+ cells that recognize viral antigen in the context of the H-2Db gene product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Jamieson B. D., Porter D. D. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987 Dec;61(12):3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman P. S., Ernst P. B., Rosenthal K. L., Clark D. A., Befus A. D., Bienenstock J. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986 Mar 1;136(5):1548–1553. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Dörries R., Watanabe R., Wege H., ter Meulen V. Murine coronavirus-induced encephalomyelitis in rats: analysis of immunoglobulins and virus-specific antibodies in serum and cerebrospinal fluid. J Neuroimmunol. 1986 Aug;12(2):131–142. doi: 10.1016/0165-5728(86)90026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Shubin R. A., Sussman M. A., Casteel N., Stohlman S. A. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology. 1989 Jan;168(1):162–167. doi: 10.1016/0042-6822(89)90415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Ting J. Y., Stohlman S. A., Weiner L. P. Improvements in obtaining and characterizing mouse cerebrospinal fluid. Application to mouse hepatitis virus-induced encephalomyelitis. J Neuroimmunol. 1983 Apr;4(2):129–140. doi: 10.1016/0165-5728(83)90017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., Bradbury J., Stohlman S. A., Weiner L. P. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb Pathog. 1987 Jul;3(1):9–20. doi: 10.1016/0882-4010(87)90033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai C., Tamura T., Fujiwara K. Effect of immune heterozygous spleen cell transfer on resistance to mouse hepatitis virus infection in nude mice. Microbiol Immunol. 1981;25(10):1011–1018. doi: 10.1111/j.1348-0421.1981.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Lampert P. W., Oldstone M. B. Virus persistence and recurring demyelination produced by a temperature-sensitive mutant of MHV-4. Nature. 1982 Jul 15;298(5871):279–280. doi: 10.1038/298279a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Suzumura A., Lampson L. A., Siegel R. M., Murasko D. M., Silberberg D. H., Weiss S. R. Expression of MHC class I genes in mouse hepatitis virus (MHV-A59) infection and in multiple sclerosis. Adv Exp Med Biol. 1987;218:219–222. doi: 10.1007/978-1-4684-1280-2_26. [DOI] [PubMed] [Google Scholar]
- Lecomte J., Cainelli-Gebara V., Mercier G., Mansour S., Talbot P. J., Lussier G., Oth D. Protection from mouse hepatitis virus type 3-induced acute disease by an anti-nucleoprotein monoclonal antibody. Brief report. Arch Virol. 1987;97(1-2):123–130. doi: 10.1007/BF01310740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Morrison L. A., Braciale V. L., Malissen B., Braciale T. J. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985 Jul 1;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., Brinkmann R., ter Meulen V. Inducibility of Ia antigen on astrocytes by murine coronavirus JHM is rat strain dependent. J Exp Med. 1987 Jul 1;166(1):259–264. doi: 10.1084/jem.166.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., Wege H., ter Meulen V. Analysis and pathogenetic significance of class II MHC (Ia) antigen induction on astrocytes during JHM coronavirus infection in rats. Adv Exp Med Biol. 1987;218:203–217. doi: 10.1007/978-1-4684-1280-2_25. [DOI] [PubMed] [Google Scholar]
- Nakanaga K., Yamanouchi K., Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986 Jul;59(1):168–171. doi: 10.1128/jvi.59.1.168-171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Perlman S., Schelper R., Bolger E., Ries D. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb Pathog. 1987 Mar;2(3):185–194. doi: 10.1016/0882-4010(87)90020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel K., Müller M. A., ter Meulen V. Influence of maternal immunity on the outcome of murine coronavirus JHM infection in suckling mice. Med Microbiol Immunol. 1985;174(1):15–24. doi: 10.1007/BF02123666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Barthold S. W., Beck D. S. Intranasally administered alpha/beta interferon prevents extension of mouse hepatitis virus, strain JHM, into the brains of BALB/cByJ mice. Antiviral Res. 1987 Dec;8(5-6):239–245. doi: 10.1016/S0166-3542(87)80002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Saravani A., Dales S. In vivo and in vitro models of demyelinating disease. XVII. The infectious process in athymic rats inoculated with JHM virus. Microb Pathog. 1987 Feb;2(2):79–90. doi: 10.1016/0882-4010(87)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz L., Baenziger J., Pircher H., Hengartner H., Zinkernagel R. M. Effect of rabbit anti-asialo GM1 treatment in vivo or with anti-asialo GM1 plus complement in vitro on cytotoxic T cell activities. J Immunol. 1986 Jun 15;136(12):4674–4680. [PubMed] [Google Scholar]
- Stohlman S. A., Brayton P. R., Harmon R. C., Stevenson D., Ganges R. G., Matsushima G. K. Natural killer cell activity during mouse hepatitis virus infection: response in the absence of interferon. Int J Cancer. 1983 Mar 15;31(3):309–314. doi: 10.1002/ijc.2910310310. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Matsushima G. K., Casteel N., Weiner L. P. In vivo effects of coronavirus-specific T cell clones: DTH inducer cells prevent a lethal infection but do not inhibit virus replication. J Immunol. 1986 Apr 15;136(8):3052–3056. [PubMed] [Google Scholar]
- Stohlman S. A., Sussman M. A., Matsushima G. K., Shubin R. A., Erlich S. S. Delayed-type hypersensitivity response in the central nervous system during JHM virus infection requires viral specificity for protection. J Neuroimmunol. 1988 Sep;19(3):255–268. doi: 10.1016/0165-5728(88)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Weiss S. R., Silberberg D. H. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986 May 23;232(4753):991–993. doi: 10.1126/science.3010460. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Goto N., Matsubara Y., Fujiwara K. Postinflammatory remyelination in the spinal cord of mice infected with mouse hepatitis virus, JHM strain. Jpn J Exp Med. 1987 Jun;57(3):145–151. [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983 Sep 8;305(5930):150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Dörries R., Wege H. Hybridoma antibodies to the murine coronavirus JHM: characterization of epitopes on the peplomer protein (E2). J Gen Virol. 1984 Nov;65(Pt 11):1931–1942. doi: 10.1099/0022-1317-65-11-1931. [DOI] [PubMed] [Google Scholar]
- Wege H., Koga M., Watanabe R., Nagashima K., ter Meulen V. Neurovirulence of murine coronavirus JHM temperature-sensitive mutants in rats. Infect Immun. 1983 Mar;39(3):1316–1324. doi: 10.1128/iai.39.3.1316-1324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Haspel M. V., Parker D. C., Holmes K. V. Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J Immunol. 1986 Feb 15;136(4):1454–1460. [PubMed] [Google Scholar]
- Welsh R. M., Haspel M. V., Parker D. C., Holmes K. V. Natural cytotoxicity against mouse hepatitis virus-infected cells. II. A cytotoxic effector cell with a B lymphocyte phenotype. J Immunol. 1986 Feb 15;136(4):1454–1460. [PubMed] [Google Scholar]
- Woodward J. G., Matsushima G., Frelinger J. A., Stohlman S. A. Production and characterization of T cell clones specific for mouse hepatitis virus, strain JHM: in vivo and in vitro analysis. J Immunol. 1984 Aug;133(2):1016–1021. [PubMed] [Google Scholar]
- Yamaguchi K., Kyuwa S., Nakanaga K., Hayami M. Establishment of cytotoxic T-cell clones specific for cells infected with mouse hepatitis virus. J Virol. 1988 Jul;62(7):2505–2507. doi: 10.1128/jvi.62.7.2505-2507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


