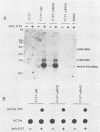
Abstract
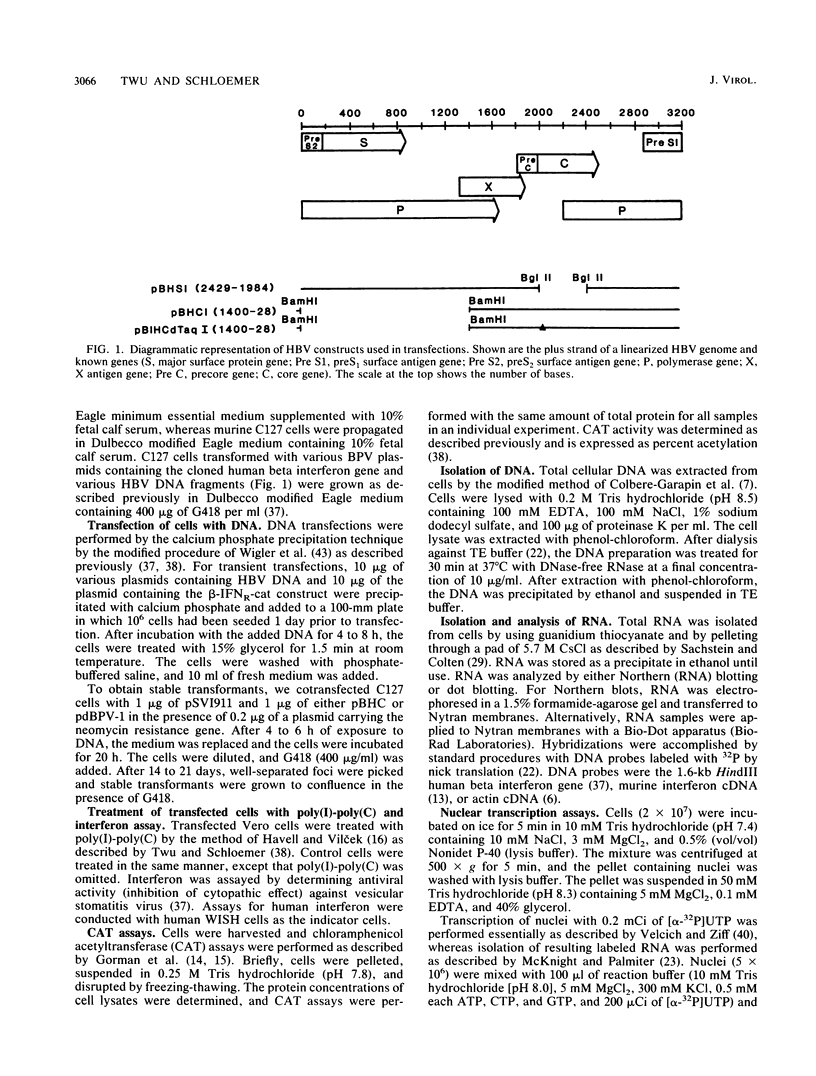
The manner by which the trans-acting factor encoded by the 1,828-base-pair (bp) BamHI DNA fragment of hepatitis B virus (HBV) suppresses the production of human beta interferon was determined. Steady-state levels of RNA specific for human beta interferon were decreased in cells that contained the 1,828-bp BamHI DNA fragment of HBV. The reduced accumulation of interferon-specific RNA was due to an inhibition of transcription of the interferon gene by the HBV trans-acting moiety. The expression of the interferon gene that is under the control of a heterologous promoter such as the simian virus 40 early promoter was not altered by the presence of the 1,828-bp BamHI HBV DNA fragment. In contrast, the HBV moiety inhibited the expression of the cat gene, whose expression is controlled by the regulatory DNA region of the human beta interferon gene. These results indicate that the HBV trans-acting moiety suppresses the expression of the human beta interferon gene at the transcriptional level by interacting with the regulatory DNA sequences 5' to the coding sequences for beta interferon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonucci T. K., Rutter W. J. Hepatitis B virus (HBV) promoters are regulated by the HBV enhancer in a tissue-specific manner. J Virol. 1989 Feb;63(2):579–583. doi: 10.1128/jvi.63.2.579-583.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavand M. R., Laub O. Two proteins with reverse transcriptase activities associated with hepatitis B virus-like particles. J Virol. 1988 Feb;62(2):626–628. doi: 10.1128/jvi.62.2.626-628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Enders G., Sprengel R., Peters N., Varmus H. E., Ganem D. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J Virol. 1987 Oct;61(10):3322–3325. doi: 10.1128/jvi.61.10.3322-3325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Mandart E., Hampe A., Fitoussi F., Tiollais P., Galibert F. Localization on the viral genome and nucleotide sequence of the gene coding for the two major polypeptides of the hepatitis B surface antigen (HBs Ag). Nucleic Acids Res. 1979 Sep 25;7(2):335–346. doi: 10.1093/nar/7.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. L., Hoofnagle J. H. Interferon in viral hepatitis: role in pathogenesis and treatment. Hepatology. 1986 Sep-Oct;6(5):1038–1041. doi: 10.1002/hep.1840060537. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. A., Walsh J. H., Purcell R. H. Failure to demonstrate circulating interferon during incubation period and acute stage of transfusion-associated hepatitis. Proc Soc Exp Biol Med. 1971 Mar;136(3):853–856. doi: 10.3181/00379727-136-35379. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Lever A. M., Thomas H. C. Evidence for a deficiency of interferon production in patients with chronic hepatitis B virus infection acquired in adult life. Hepatology. 1986 Sep-Oct;6(5):962–965. doi: 10.1002/hep.1840060525. [DOI] [PubMed] [Google Scholar]
- Kinoshita R., Miura K., Suzuki S., Uchino H. Lack of correlation between interferon production of mononuclear cells and virus replication in chronic hepatitis B virus infection. J Clin Microbiol. 1979 Dec;10(6):923–925. doi: 10.1128/jcm.10.6.923-925.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay P., Lees J., Murray K. The conversion of hepatitis B core antigen synthesized in E coli into e antigen. J Med Virol. 1981;8(4):237–243. doi: 10.1002/jmv.1890080404. [DOI] [PubMed] [Google Scholar]
- Malpièce Y., Michel M. L., Carloni G., Revel M., Tiollais P., Weissenbach J. The gene S promoter of hepatitis B virus confers constitutive gene expression. Nucleic Acids Res. 1983 Jul 11;11(13):4645–4654. doi: 10.1093/nar/11.13.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano L., Miescher G. C., Goodall A. H., Wiedmann K. H., Janossy G., Thomas H. C. Hepatitis B virus and HLA antigen display in the liver during chronic hepatitis B virus infection. Hepatology. 1982 Sep-Oct;2(5):557–561. doi: 10.1002/hep.1840020508. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitrine A., Chousterman S., Chousterman M., Naveau S., Thang M. N., Chaput J. C. Lack of in vivo activation of the interferon system in HBsAg-positive chronic active hepatitis. Hepatology. 1985 Mar-Apr;5(2):171–174. doi: 10.1002/hep.1840050202. [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackstein R., Colten H. R. Molecular regulation of MHC class III (C4 and factor B) gene expression in mouse peritoneal macrophages. J Immunol. 1984 Sep;133(3):1618–1626. [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Kronenberg L. H., Rosenblatt H. M., Bryson Y., Merigan T. C. UCLA conference. Interferon: immunobiology and clinical significance. Ann Intern Med. 1982 Jan;96(1):80–93. doi: 10.7326/0003-4819-96-1-80. [DOI] [PubMed] [Google Scholar]
- Taylor P. E., Zuckerman A. J. Non-production of interfering substances by serum from patients with infectious hepatitis. J Med Microbiol. 1968 Nov;1(2):217–219. doi: 10.1099/00222615-1-2-217. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tolentino P., Dianzani F., Zucca M., Giacchino R. Decreased interferon response by lymphocytes from children with chronic hepatitis. J Infect Dis. 1975 Oct;132(4):459–461. doi: 10.1093/infdis/132.4.459. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Lee C. H., Lin P. M., Schloemer R. H. Hepatitis B virus suppresses expression of human beta-interferon. Proc Natl Acad Sci U S A. 1988 Jan;85(1):252–256. doi: 10.1073/pnas.85.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Hallenbeck L. A. Effect of virus infections on target cell susceptibility to natural killer cell-mediated lysis. J Immunol. 1980 May;124(5):2491–2497. [PubMed] [Google Scholar]
- Wheelock E. F., Schenker S., Combes B. Absence of circulating interferon in patients with infectious and serum hepatitis. Proc Soc Exp Biol Med. 1968 May;128(1):251–253. doi: 10.3181/00379727-128-32989. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]