Abstract
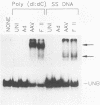
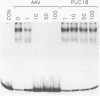
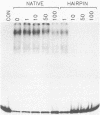
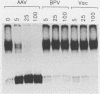
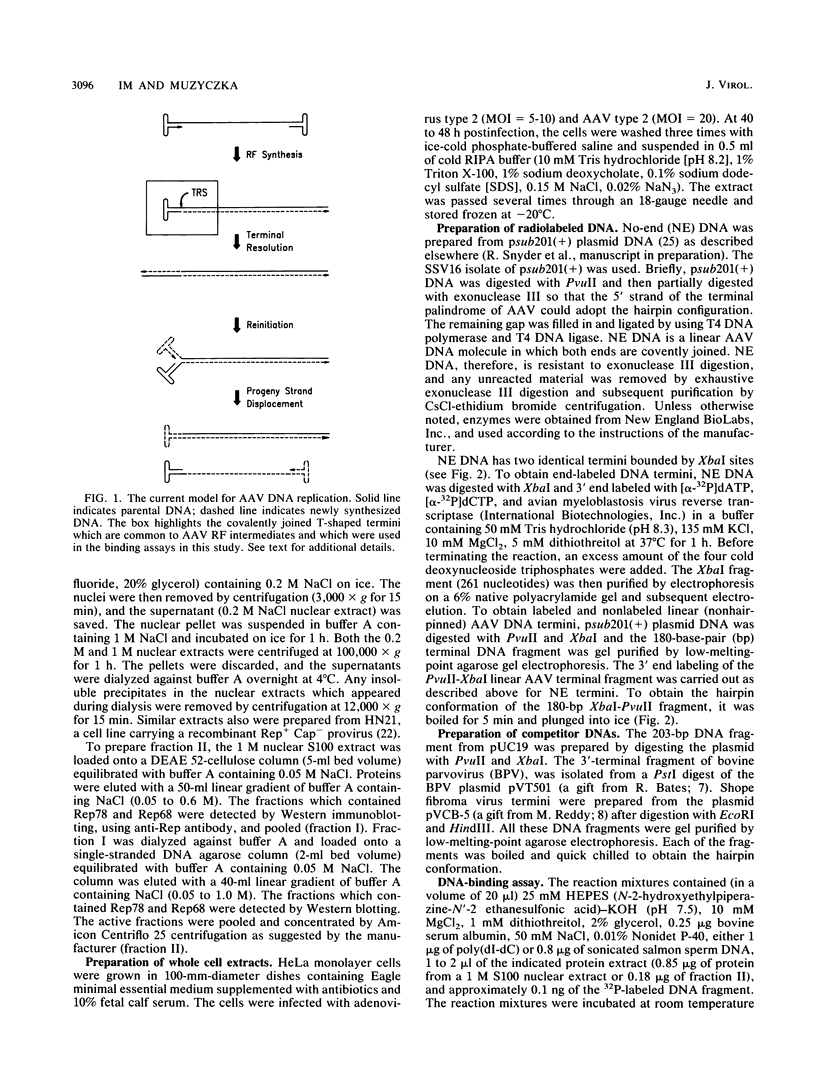
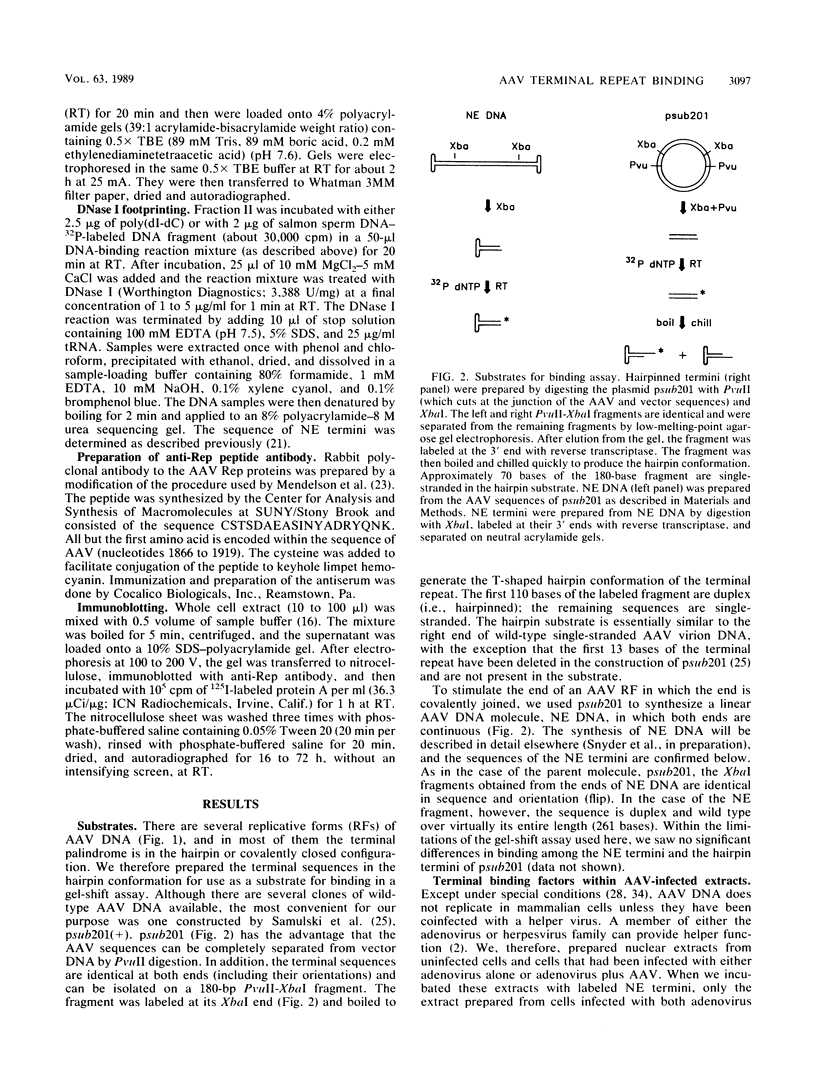
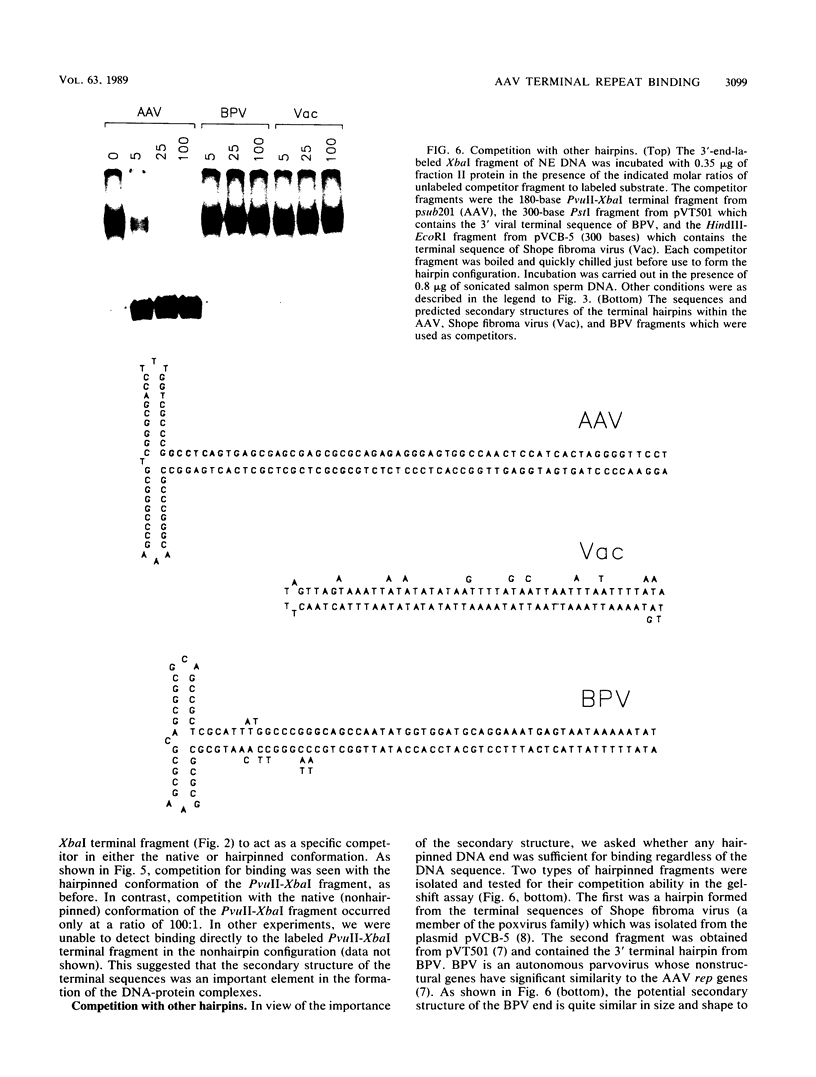
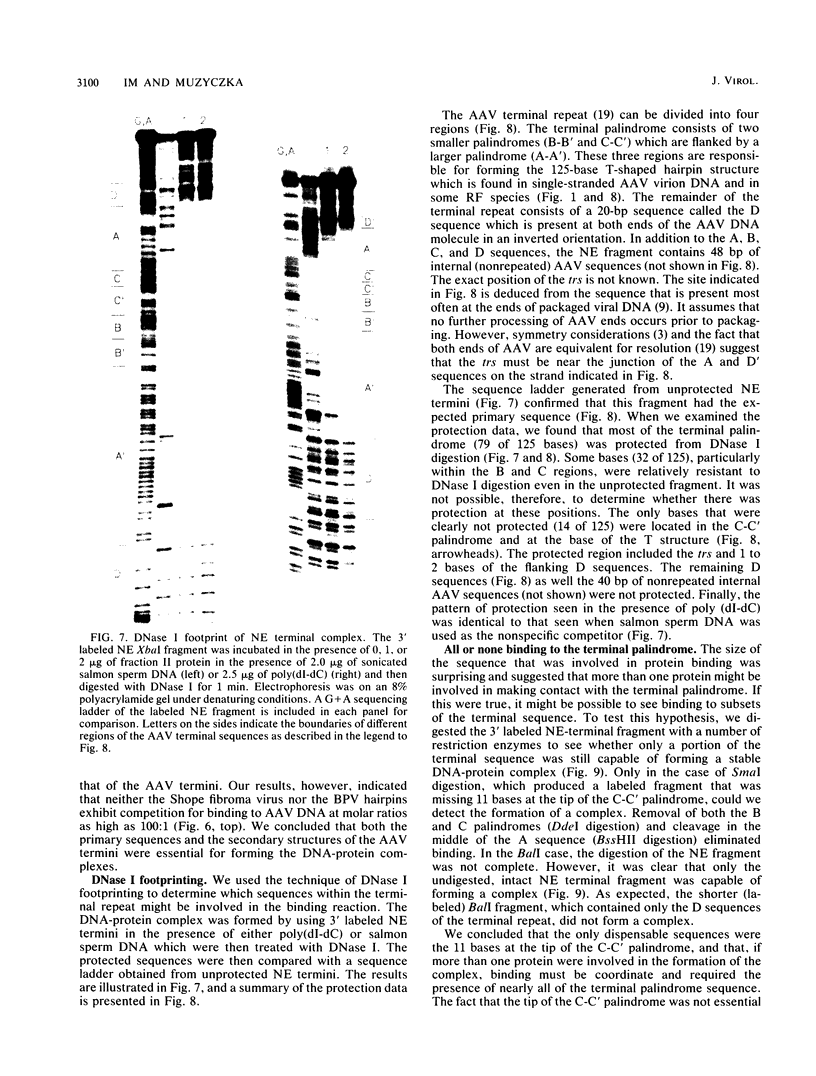
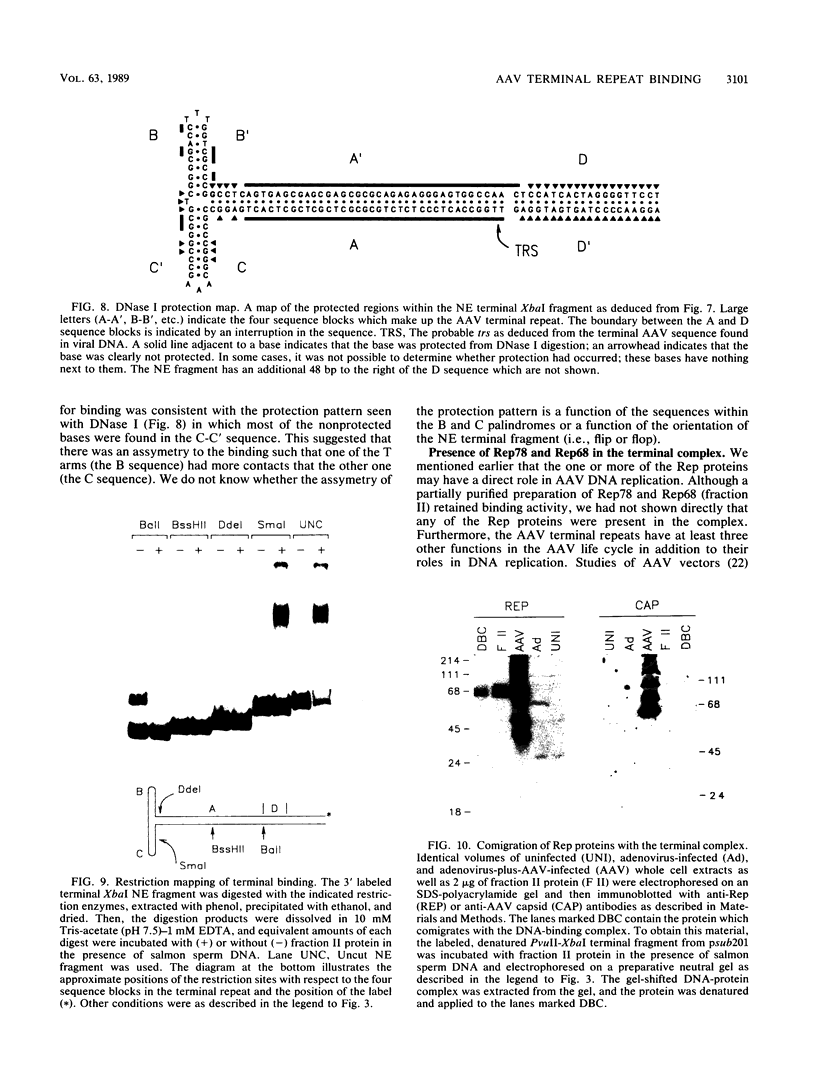
We have identified and characterized a DNA-protein complex that forms with the adeno-associated virus (AAV) terminal repeats. The complex formed only if the terminal palindrome was in the covalently closed or hairpin configuration; little if any binding was detected with the open duplex form of the terminal repeat. This fact suggested that both secondary structure and primary sequence are essential elements of recognition. DNase I protection studies indicated that virtually all of the A-A' palindrome and significant portions of the B-B' and C-C' palindromes are protected. The postulated terminal resolution site of AAV also is protected. Restriction mapping of the sequences necessary for binding indicated that almost all of the terminal palindrome must be present for binding to occur. Hairpins which are similar in size and shape to the AAV termini did not exhibit competition for binding, and the complex formed only if AAV-infected extracts were used. Thus, the binding reaction is specific for AAV sequences. The viral-coded nonstructural proteins Rep78 and Rep68 comigrated with the DNA-protein complex on neutral acrylamide gels, suggesting that one or both of these proteins are components of the complex. The characteristics of the complex suggested that it has a role in AAV DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Chow M. B., Ward D. C. Sequence analysis of the termini of virion and replicative forms of minute virus of mice DNA suggests a modified rolling hairpin model for autonomous parvovirus DNA replication. J Virol. 1985 Apr;54(1):171–177. doi: 10.1128/jvi.54.1.171-177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- Bohenzky R. A., LeFebvre R. B., Berns K. I. Sequence and symmetry requirements within the internal palindromic sequences of the adeno-associated virus terminal repeat. Virology. 1988 Oct;166(2):316–327. doi: 10.1016/0042-6822(88)90502-8. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., Reddy M., Scraba D., Upton C., McFadden G. Replication and resolution of cloned poxvirus telomeres in vivo generates linear minichromosomes with intact viral hairpin termini. J Virol. 1986 Aug;59(2):249–259. doi: 10.1128/jvi.59.2.249-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife K. H., Berns K. I., Murray K. Structure and nucleotide sequence of the terminal regions of adeno-associated virus DNA. Virology. 1977 May 15;78(2):475–477. doi: 10.1016/0042-6822(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb J., Muzyczka N. In vitro excision of adeno-associated virus DNA from recombinant plasmids: isolation of an enzyme fraction from HeLa cells that cleaves DNA at poly(G) sequences. Mol Cell Biol. 1988 Jun;8(6):2513–2522. doi: 10.1128/mcb.8.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Berns K. I. The adeno-associated virus rep gene inhibits replication of an adeno-associated virus/simian virus 40 hybrid genome in cos-7 cells. J Virol. 1988 May;62(5):1705–1712. doi: 10.1128/jvi.62.5.1705-1712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Hermonat P. L., Berns K. I. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J Virol. 1986 Oct;60(1):251–258. doi: 10.1128/jvi.60.1.251-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughlin C. A., Cardellichio C. B., Coon H. C. Latent infection of KB cells with adeno-associated virus type 2. J Virol. 1986 Nov;60(2):515–524. doi: 10.1128/jvi.60.2.515-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby E., Bohenzky R., Berns K. I. Inverted terminal repetition in adeno-associated virus DNA: independence of the orientation at either end of the genome. J Virol. 1981 Mar;37(3):1083–1086. doi: 10.1128/jvi.37.3.1083-1086.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby E., Fife K. H., Berns K. I. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980 May;34(2):402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. K., Collis P., Hermonat P. L., Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988 Jun;62(6):1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson E., Trempe J. P., Carter B. J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986 Dec;60(3):823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Berns K. I., Tan M., Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987 Oct;61(10):3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Tratschin J. D., Carter B. J. Replication of adeno-associated virus DNA. Complementation of naturally occurring rep- mutants by a wild-type genome or an ori- mutant and correction of terminal palindrome deletions. J Mol Biol. 1984 Oct 15;179(1):1–20. doi: 10.1016/0022-2836(84)90303-6. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]