Abstract
Sequence analyses of the left and right termini of LuIII virus show they are nonidentical imperfect palindromes of 122 and 211 nucleotides, respectively. The left terminus of the minus strand of LuIII DNA, uniquely in the flip conformation, can assume a T-shaped structure. The right terminus of the minus strand of LuIII DNA can assume a U-shaped structure, and it exists in either the flip or flop conformation. The termini of LuIII shared a high degree of sequence homology and showed conserved secondary structure with those of the rodent parvoviruses MVMp and H-1. LuIII, like adeno-associated virus, encapsidates equal amounts of plus- and minus-strand DNA. However, the sequence data for LuIII virus demonstrate that identical termini are not required for this encapsidation pattern.
Full text
PDF
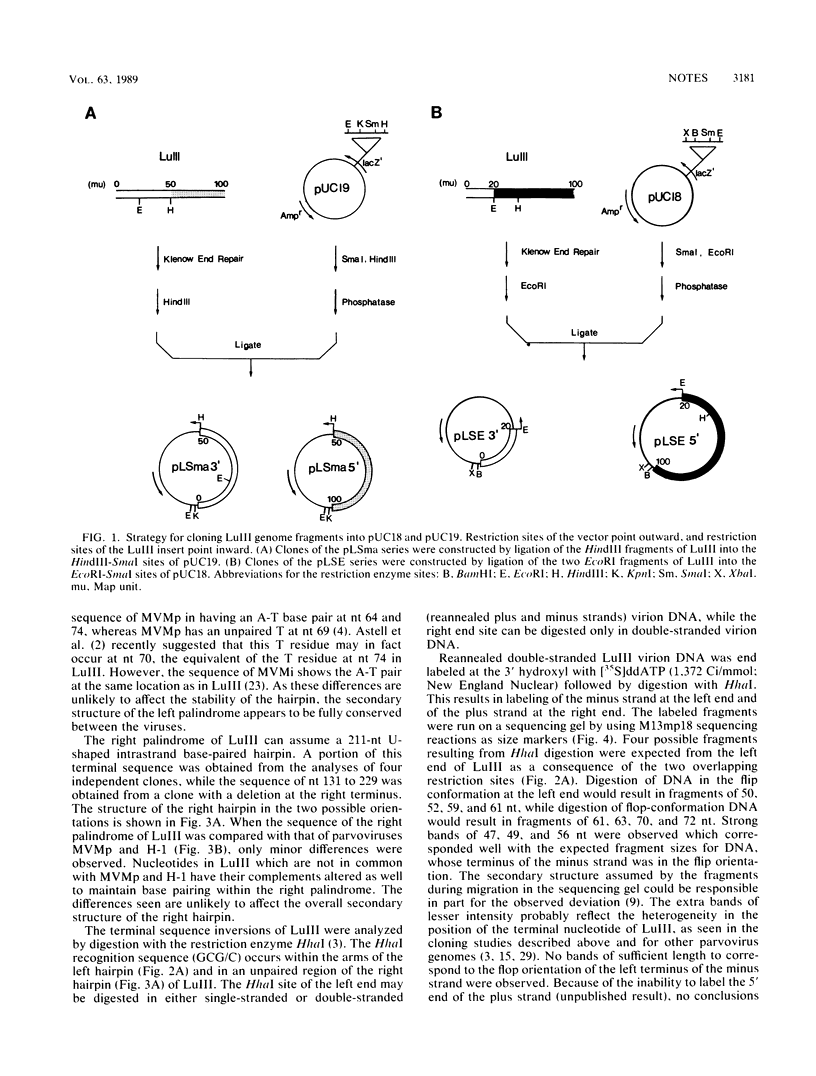
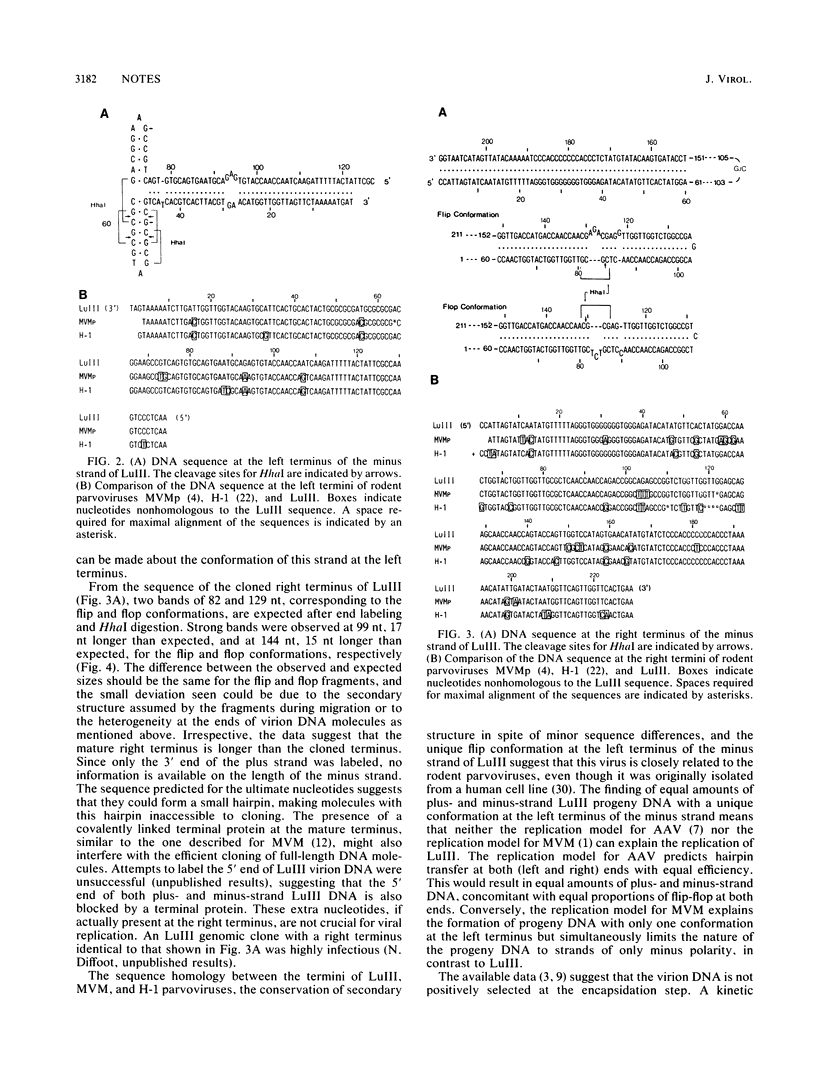


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Chow M. B., Ward D. C. Sequence analysis of the termini of virion and replicative forms of minute virus of mice DNA suggests a modified rolling hairpin model for autonomous parvovirus DNA replication. J Virol. 1985 Apr;54(1):171–177. doi: 10.1128/jvi.54.1.171-177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Gardiner E. M., Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986 Feb;57(2):656–669. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Smith M., Chow M. B., Ward D. C. Structure of the 3' hairpin termini of four rodent parvovirus genomes: nucleotide sequence homology at origins of DNA replication. Cell. 1979 Jul;17(3):691–703. doi: 10.1016/0092-8674(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Snyder C. E., Banerjee P. T., Mitra S. Autonomous parvovirus LuIII encapsidates equal amounts of plus and minus DNA strands. J Virol. 1984 Feb;49(2):319–324. doi: 10.1128/jvi.49.2.319-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Lederman M., Stout E. R., Bates R. C. Analysis of the termini of the DNA of bovine parvovirus: demonstration of sequence inversion at the left terminus and its implication for the replication model. J Virol. 1988 Oct;62(10):3807–3813. doi: 10.1128/jvi.62.10.3807-3813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife K. H., Berns K. I., Murray K. Structure and nucleotide sequence of the terminal regions of adeno-associated virus DNA. Virology. 1977 May 15;78(2):475–477. doi: 10.1016/0042-6822(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Klaassen B. DNA sequence of the 5' terminus containing the replication origin of parvovirus replicative form DNA. J Virol. 1982 Mar;41(3):990–999. doi: 10.1128/jvi.41.3.990-999.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. X. Isolation of a mutant defective in replicative-form DNA replication. J Virol. 1978 Jan;25(1):215–223. doi: 10.1128/jvi.25.1.215-223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Sahli R., McMaster G. K., Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985 May 24;13(10):3617–3633. doi: 10.1093/nar/13.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., Fabisch P. Nucleotide sequence of the self-priming 3' terminus of the single-stranded DNA extracted from the parvovirus Kilham rat virus. J Virol. 1979 Jun;30(3):946–950. doi: 10.1128/jvi.30.3.946-950.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Srivastava A., Berns K. I., Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983 May;33(1):135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull B. C., Chen K. C., Lederman M., Stout E. R., Bates R. C. Genomic clones of bovine parvovirus: construction and effect of deletions and terminal sequence inversions on infectivity. J Virol. 1988 Feb;62(2):417–426. doi: 10.1128/jvi.62.2.417-426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Summers J., Jones S. E., Anderson M. J. Characterization of the genome of the agent of erythrocyte aplasia permits its classification as a human parvovirus. J Gen Virol. 1983 Nov;64(Pt 11):2527–2532. doi: 10.1099/0022-1317-64-11-2527. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Molecular structure of adeno-associated virus variant DNA. J Biol Chem. 1980 Apr 10;255(7):3194–3203. [PubMed] [Google Scholar]