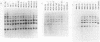Abstract
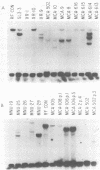
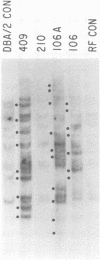
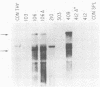
RF/J mice are susceptible to the induction of thymic lymphomas by the carcinogens 3-methylcholanthrene and N-methyl-N-nitrosourea. Given the association of mouse mammary tumor virus (MMTV) with certain thymomas, we examined genomic DNA from chemically induced lymphomas of RF/J mice for new MMTV proviruses. Of 13 tissue culture lines derived from 3-methylcholanthrene-induced tumors, 5 had acquired new proviruses. MMTV amplification coincided with the appearance of viral mRNAs and proteins. However, no primary tumors or animal-passaged tumors contained new proviruses. These observations indicate that MMTV does not have a role in the tumor induction process, although it may become activated and amplified in tissue culture lines derived from tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J. K., Arthur L. O., Dekaban G. A. The involvement of a type-B retrovirus in the induction of thymic lymphomas. Virology. 1985 Jan 15;140(1):159–172. doi: 10.1016/0042-6822(85)90455-6. [DOI] [PubMed] [Google Scholar]
- Ball J. K., Dekaban G. A., McCarter J. A., Loosmore S. M. Molecular biological characterization of a highly leukaemogenic virus isolated from the mouse. III. Identity with mouse mammary tumour virus. J Gen Virol. 1983 Oct;64(Pt 10):2177–2190. doi: 10.1099/0022-1317-64-10-2177. [DOI] [PubMed] [Google Scholar]
- Chinsky J., Goodenow M. M., Jackson M., Lilly F., Leinwand L., Childs G. Comparison of endogenous murine leukemia virus proviral organization and RNA expression in 3-methylcholanthrene-induced and spontaneous thymic lymphomas in RF and AKR mice. J Virol. 1985 Jan;53(1):94–99. doi: 10.1128/jvi.53.1.94-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Lilly F., Childs G. Comparison of chemically induced and spontaneous murine thymic lymphomas in RF and AKR mice: differential expression of c-myc and c-myb. Proc Natl Acad Sci U S A. 1985 Jan;82(2):565–569. doi: 10.1073/pnas.82.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta U. B., Lilly F. Chemically induced murine T lymphomas: continued rearrangement within the T-cell receptor beta-chain gene during serial passage. Proc Natl Acad Sci U S A. 1988 May;85(9):3193–3197. doi: 10.1073/pnas.85.9.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban G. A., Ball J. K. Integration of type B retroviral DNA in virus-induced primary murine thymic lymphomas. J Virol. 1984 Dec;52(3):784–792. doi: 10.1128/jvi.52.3.784-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. E., Berman J. W., Pellicer A. Differential expression of surface markers on thymic lymphomas induced by two carcinogenic agents in different mouse strains. Cell Immunol. 1987 Jun;107(1):115–120. doi: 10.1016/0008-8749(87)90271-1. [DOI] [PubMed] [Google Scholar]
- Dudley J. P., Arfsten A., Hsu C. L., Kozak C., Risser R. Molecular cloning and characterization of mouse mammary tumor proviruses from a T-cell lymphoma. J Virol. 1986 Jan;57(1):385–388. doi: 10.1128/jvi.57.1.385-388.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P. Mouse mammary tumor proviruses from a T-cell lymphoma are associated with the retroposon L1Md. J Virol. 1988 Feb;62(2):472–478. doi: 10.1128/jvi.62.2.472-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J., Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984 Jan;49(1):92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Reynals M. L., Lilly F., Bosch A., Blank K. J. The genetic basis of susceptibility to leukemia induction in mice by 3-methylcholanthrene applied percutaneously. J Exp Med. 1978 Feb 1;147(2):459–469. doi: 10.1084/jem.147.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Wellinger R., Vessaz A., Diggelmann H. A new site of integration for mouse mammary tumor virus proviral DNA common to BALB/cf(C3H) mammary and kidney adenocarcinomas. EMBO J. 1986 Jan;5(1):127–134. doi: 10.1002/j.1460-2075.1986.tb04186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow M. M., Lilly F. Expression of differentiation and murine leukemia virus antigens on cells of primary tumors and cell lines derived from chemically induced lymphomas of RF/J mice. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7612–7616. doi: 10.1073/pnas.81.23.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow M., Kessler K., Leinwand L., Lilly F. Absence of trisomy 15 in chemically induced murine T-cell lymphomas. Cancer Genet Cytogenet. 1986 Jan 15;19(3-4):205–211. doi: 10.1016/0165-4608(86)90048-8. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Pellicer A. Mutational activation of oncogenes in animal model systems of carcinogenesis. Mutat Res. 1987 May;185(3):293–308. doi: 10.1016/0165-1110(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka S. T., Lilly F. Genetically determined resistance to 3-methylcholanthrene-induced lymphoma is expressed at the level of bone marrow-derived cells. J Exp Med. 1987 Aug 1;166(2):565–570. doi: 10.1084/jem.166.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Prakash O., Klein D., Sarkar N. H. Structural alterations in the long terminal repeat of an acquired mouse mammary tumor virus provirus in a T-cell leukemia of DBA/2 mice. Virology. 1987 Jul;159(1):39–48. doi: 10.1016/0042-6822(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Morris V. L., Flintoff W. F., Kozak C. A. Characterization and chromosomal location of endogenous mouse mammary tumor virus loci in GR, NFS, and DBA mice. Virology. 1984 Jan 15;132(1):12–25. doi: 10.1016/0042-6822(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Hilkens J., Hilgers J., Groner B., Hynes N. E. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982 Sep;43(3):819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Weijers P. Rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in T-cell leukemias of mouse strain GR result in a novel enhancer-like structure. Mol Cell Biol. 1985 Apr;5(4):823–830. doi: 10.1128/mcb.5.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., van der Ploeg L., van Duijn L., Michalides R., Hilgers J. Impaired maturation of mouse mammary tumor virus precursor polypeptides in lymphoid leukemia cells, producing intracytoplasmic A particles and no extracellular B-type virions. J Virol. 1979 Oct;32(1):251–258. doi: 10.1128/jvi.32.1.251-258.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Beyer H. Amplification of mouse mammary tumor virus genomes in non-mammary tumor cells. J Virol. 1989 Jan;63(1):456–459. doi: 10.1128/jvi.63.1.456-459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Long C. A., Sheffield J. B., Tamura A., Tanaka H. Murine mammary tumor virus deficient in the major glycoprotein: biochemical and biological studies on virions produced by a lymphoma cell line. Virology. 1980 Jul 30;104(2):279–293. doi: 10.1016/0042-6822(80)90333-5. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Garcia M., Vessaz A., Diggelmann H. Exogenous mouse mammary tumor virus proviral DNA isolated from a kidney adenocarcinoma cell line contains alterations in the U3 region of the long terminal repeat. J Virol. 1986 Oct;60(1):1–11. doi: 10.1128/jvi.60.1.1-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]