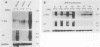Abstract
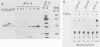
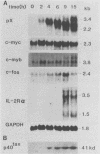
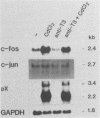
We examined the ability of the trans-acting factor p40tax of human T-cell leukemia virus type I (HTLV-I), which is thought to be a crucial molecule in T-cell transformation by HTLV-I, to activate expression of a set of endogenous cellular genes related to T-cell proliferation. For this purpose we established a subclone (JPX-9) of Jurkat cells that was stably transfected with an expression plasmid containing the p40tax gene, whose expression is definitely dependent on heavy-metal ions. Expression of the interleukin-2 receptor alpha chain in JPX-9 cells was induced in response to the induction of p40tax expression, as has been demonstrated by others in transient transfection experiments with Jurkat cells. In addition, we found that significant enhancement of expression of the nuclear proto-oncogene c-fos was closely associated with expression of p40tax. Continous enhancement in the level of c-fos mRNA was observed in the presence of p40tax. In contrast, mRNA levels of other nuclear proto-oncogenes (c-myc, c-myb, and c-jun) were not appreciably effected by the expression of p40tax. These results suggest that (i) in addition to the interleukin-2-interleukin-2 receptor system, cellular genes such as c-fos, which regulate normal T-cell growth, are also activated directly or indirectly by p40tax and (ii) p40tax-induced modulation of gene expression plays a crucial role in T-cell transformation by HTLV-I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Arima N., Daitoku Y., Ohgaki S., Fukumori J., Tanaka H., Yamamoto Y., Fujimoto K., Onoue K. Autocrine growth of interleukin 2-producing leukemic cells in a patient with adult T cell leukemia. Blood. 1986 Sep;68(3):779–782. [PubMed] [Google Scholar]
- Arya S. K., Wong-Staal F., Gallo R. C. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science. 1984 Mar 9;223(4640):1086–1087. doi: 10.1126/science.6320374. [DOI] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Bergmann D. G., Souza L. M., Baluda M. A. Vertebrate DNAs contain nucleotide sequences related to the transforming gene of avian myeloblastosis virus. J Virol. 1981 Nov;40(2):450–455. doi: 10.1128/jvi.40.2.450-455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Fujii M., Sassone-Corsi P., Verma I. M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Hattori T., Uchiyama T., Toibana T., Takatsuki K., Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981 Sep;58(3):645–647. [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Kanehisa M., Krönke M., Peffer N. J., Svetlik P. B., Sullivan M., Greene W. C. Structure of the human interleukin-2 receptor gene. Science. 1985 Nov 8;230(4726):633–639. doi: 10.1126/science.2996141. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Ohtani K., Nakamura M., Saito S., Nagata K., Sugamura K., Hinuma Y. Electroporation: application to human lymphoid cell lines for stable introduction of a transactivator gene of human T-cell leukemia virus type I. Nucleic Acids Res. 1989 Feb 25;17(4):1589–1604. doi: 10.1093/nar/17.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K., Nakamura M., Saito S., Noda T., Ito Y., Sugamura K., Hinuma Y. Identification of two distinct elements in the long terminal repeat of HTLV-I responsible for maximum gene expression. EMBO J. 1987 Feb;6(2):389–395. doi: 10.1002/j.1460-2075.1987.tb04767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskalis H., Felber B. K., Pavlakis G. N. Cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller W., Sumida T., Tokuhisa T., Rajewsky K., Wagner E. F. c-fos expression interferes with thymus development in transgenic mice. Cell. 1988 Jun 17;53(6):847–856. doi: 10.1016/s0092-8674(88)90289-9. [DOI] [PubMed] [Google Scholar]
- Sabe H., Tanaka A., Siomi H., Koyasu S., Yonehara S., Yahara I., Tagaya Y., Sugie K., Teshigawara K., Yodoi J. Differential effects on expression of IL-2 receptors (p55 and p70) by the HTLV-I pX DNA. Int J Cancer. 1988 Jun 15;41(6):880–885. doi: 10.1002/ijc.2910410619. [DOI] [PubMed] [Google Scholar]
- Saito S., Nakamura M., Ohtani K., Ichijo M., Sugamura K., Hinuma Y. trans-activation of the simian virus 40 enhancer by a pX product of human T-cell leukemia virus type I. J Virol. 1988 Feb;62(2):644–648. doi: 10.1128/jvi.62.2.644-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitani M., Nakamura M., Fujii M., Sugamura K., Hinuma Y. High affinity interleukin 2 receptors in HTLV-1-infected T cells can mediate signals for gene expression. Virus Genes. 1987 Nov;1(1):35–47. doi: 10.1007/BF00125684. [DOI] [PubMed] [Google Scholar]
- Sambucetti L. C., Curran T. The Fos protein complex is associated with DNA in isolated nuclei and binds to DNA cellulose. Science. 1986 Dec 12;234(4782):1417–1419. doi: 10.1126/science.3491427. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Kamps M., Verma I. M. fos-associated cellular p39 is related to nuclear transcription factor AP-1. Cell. 1988 Aug 12;54(4):553–560. doi: 10.1016/0092-8674(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Seiki M., Eddy R., Shows T. B., Yoshida M. Nonspecific integration of the HTLV provirus genome into adult T-cell leukaemia cells. Nature. 1984 Jun 14;309(5969):640–642. doi: 10.1038/309640a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Goh W. C., Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- Stern J. B., Smith K. A. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986 Jul 11;233(4760):203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Kannagi M., Sakitani M., Takeuchi M., Hinuma Y. Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer. 1984 Aug 15;34(2):221–228. doi: 10.1002/ijc.2910340213. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tozawa H., Hayami M., Sugamura K., Hinuma Y. Distinct reactivities of four monoclonal antibodies with human interleukin 2 receptor. Microbiol Immunol. 1985;29(10):959–972. doi: 10.1111/j.1348-0421.1985.tb02960.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Hori T., Tsudo M., Wano Y., Umadome H., Tamori S., Yodoi J., Maeda M., Sawami H., Uchino H. Interleukin-2 receptor (Tac antigen) expressed on adult T cell leukemia cells. J Clin Invest. 1985 Aug;76(2):446–453. doi: 10.1172/JCI111992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten F., Müller R., Curran T., Van Beveren C., Verma I. M. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]