Abstract
Hyperacute rejection of pig organs by humans involves the interaction of Galα(1,3)Gal with antibodies and complement. Strategies to reduce the amount of xenoantigen Galα(1,3)Gal were investigated by overexpression of human lysosomal α-galactosidase in cultured porcine cells and transgenic mice. The overexpression of human α-galactosidase in cultured porcine endothelial cells and COS cells resulted in a 30-fold reduction of cell surface Galα(1,3)Gal and a 10-fold reduction in cell reactivity with natural human antibodies. Splenocytes from transgenic mice overexpressing human α-galactosidase showed only a 15–25% reduction in binding to natural human anti-Galα(1,3)Gal antibodies; however, this decrease was functionally significant as demonstrated by reduced susceptibility to human antibody-mediated lysis. However, because there is residual Galα(1,3)Gal and degalactosylation results in the exposure of N-acetyllactosamine residues and potential new xenoepitopes, using α-galactosidase alone is unlikely to overcome hyperacute rejection. We previously reported that mice overexpressing human α1,2-fucosyltransferase as a transgene had ≈90% reduced Galα(1,3)Gal levels due to masking of the xenoantigen by fucosylation; we evaluated the effect of overexpressing α-galactosidase and α1,2-fucosyltransferase on Galα(1,3)Gal levels. Galα(1,3)Gal-positive COS cells expressing α1,3-galactosyltransferase, α1,2-fucosyltransferase, and α-galactosidase showed negligible cell surface staining and were not susceptible to lysis by human serum containing antibody and complement. Thus, α1,2-fucosyltransferase and α-galactosidase effectively reduced the expression of Galα(1,3)Gal on the cell surface and could be used to produce transgenic pigs with negligible levels of cell surface Galα(1,3)Gal, thereby having no reactivity with human serum and improving graft survival.
A major obstacle to xenotransplantation of pig organs into humans is the presence of natural human IgG and IgM antibodies that react with molecules on pig cells, particularly pig endothelial cells in vascularized organs, and cause hyperacute rejection (HAR) (1–3). It is now generally accepted that all or most of this reaction is caused by the presence in humans of large amounts of antibodies to the carbohydrate epitope Galα(1,3)Gal (4). The presence of the Galα(1,3)Gal epitope has been shown by absorption studies, particularly with Gal+ transfected cells but also by the demonstration that Galα(1,3)Gal carbohydrates can block the reaction both in vitro and in vivo (3).
Attempts to prevent hyperacute rejection include the removal or neutralization of complement by using cobra venom factor or by making transgenic pigs expressing human complement regulatory molecules such as CD46, CD55, and CD59. Unfortunately, these efforts have resulted in limited graft protection (5). Other means to overcome hyperacute rejection include the removal of antibody, which, although logistically difficult, leads to prolonged graft survival in pig-to-baboon transplantations (2, 6). Other means of preventing the expression of the Galα(1,3)Gal gene have been suggested (1) and include the use of anti-sense constructs, either as oligonucleotides or as cDNA, but these also have met with disappointing results (7). Another approach is to modify the Galα(1,3)Gal antigen itself, and we previously have described the isolation of the gene encoding the pig α1,3-galactosyltransferase (8), with the aim of performing gene knockout studies by homologous recombination. However, such knockout procedures have not been done in the pig (9).
We now describe the successful in vitro and in vivo reduction of Galα(1,3)Gal by the expression of the enzyme α-galactosidase, which cleaves terminal α-linked galactosyl residues on oligosaccharides. In vitro treatment of pig endothelial cells, lymphocytes, or rabbit erythrocytes with α-galactosidase totally eradicated their reaction with human natural antibodies (10–14). The enzyme also has been perfused into organs before transplantation (10). However, using soluble enzyme is difficult; the enzyme is expensive, and perfusion before transplantation would be unlikely to totally eradicate Galα(1,3)Gal. We report herein that transfection of mammalian cells with the human α-galactosidase cDNA resulted in a substantial reduction in Galα(1,3)Gal expression. However the transfected cells exposed subterminal sugars to which there were also natural antibodies. This obstacle was successfully overcome when α-galactosidase was coexpressed with α1,2-fucosyltransferase, resulting in cells that were phenotypically Galα(1,3)Gal−.
MATERIALS AND METHODS
Hemagglutination to Detect Cell Surface Galα(1,3)Gal.
Rabbit erythrocytes (Galα(1,3)Gal+) were prepared as a 2% (vol/vol) suspension in isotonic phosphate–citrate–sodium chloride (pH 5.6) and either were treated or not with purified human α-galactosidase (15) or Escherichia coli-derived α-galactosidase (Boehringer Mannheim) for 2 h at 37°C. The cells were then washed, and hemagglutination was performed by incubating dilutions of IB4 lectin [isolated from Griffonia simplicifolia, specific for Galα(1,3)Gal; Sigma] (16) in 50 μl in microtiter plates with 50-μl aliquots of α-galactosidase-treated or untreated erythrocytes. The cells were incubated for 30 min at 37°C followed by 30 min on ice. The end-point (50% hemagglutination) titer was determined by microscopy. The units of α-galactosidase activity were determined by using recombinant human α-galactosidase as standard where 1 unit of activity is the amount of enzyme that hydrolyzed 1 nmol of 4-methylumbelliferyl-α-d-galactopyranoside per hour (15).
cDNAs, Transfection, and Serology.
The plasmids used were: human α-galactosidase cDNA (15); porcine α1,3-galactosyltransferase (8) and human CD48 cDNA (17); and human α1,2-fucosyltransferase cDNA (18), all prepared by using standard techniques (19). COS-7 cells were maintained in DMEM (Trace Biosciences, Castle Hill, Australia) and were transfected (5 μg DNA/10 cm dish) by using DEAE–Dextran (17) in DMEM supplemented with 10% Nu-Serum (Collaborative Research, Bedford, MA); 48 h later, cells were examined by fluorescence microscopy. The pig endothelial cell line PIEC (a gift from K. Welsh, Churchill Hospital Oxford, U.K.) was cultured in DMEM supplemented with 10% fetal bovine serum. PIEC expressing human α-galactosidase were produced by calcium phosphate transfection (19) of α-galactosidase cDNA (20 μg), selecting for stable integration in media containing G418 (1 mg/ml; GIBCO/BRL). Detection of Galα(1,3)Gal was performed with fluorescein isothiocyanate (FITC)-conjugated IB4 lectin or the polyclonal chicken anti-laminin antibody (Austin Research Institute) and FITC-conjugated goat anti-chicken IgG (Silenus, Paris). For the binding of human natural antibodies, cells were incubated with a dilution of 1:10 of pooled human serum purified on a column of Galα(1,3)Gal bound to a glass matrix column (Syntesome, Munich, Germany) or with a dilution of 1:10 of the unbound fraction. H substance (the universally tolerated O blood group antigen) was detected with FITC-conjugated UEA1 lectin (Sigma). An mAb specific for CD48 (ASH1360, Austin Research Institute) and FITC-conjugated sheep anti-mouse IgG (Silenus) were used for cell surface staining of CD48 in control studies. Flow cytometry analyses were performed with a Becton Dickinson FACScan cytometer, and data were collected on 2.5–5 × 103 cells. Transfected cells were assayed for α-galactosidase activity by using p-nitrophenyl-α-d-galactoside as the substrate (20). Protein concentrations were determined by Bradford assay by using BSA as standard (21).
Complement Lysis Assay.
Complement-mediated lysis assays of cells transfected with cDNAs for α-galactosidase A, α1,3-galactosyltransferase, and α1,2-fucosyltransferase were performed (22). In brief, cells (50 μl) at 5 × 106/ml were mixed with 50 μl of heat-inactivated human serum (serial dilutions), incubated for 30 min at 4°C, and washed once, and 50 μl of rabbit complement (1/14 dilution) was added and incubated at 37°C for 30 min. Cell lysis was determined microscopically by using aniline blue dye exclusion.
Production and Screening of Transgenic Mice.
A 1,320-bp NruI/NotI DNA fragment encoding human α-galactosidase cDNA was generated by using the PCR, human α-galactosidase cDNA (15), and two primers: 5′-GCGAATTCTCGCGAATGCAGCTGAGGAACCCAGAACTACA, in which the underlined sequence contains a unique NruI site, and 3′-GCCTGCAGGCGGCCGCTTAAAGTAAGTCTTTTAATGACATCTGCAT, in which the underlined sequence contains a unique NotI site. The DNA was purified and directionally subcloned into exon 1 of the murine H-2Kb gene (23). The construct was engineered such that translation would begin at the ATG initiation codon of the human α-galactosidase cDNA and terminate at the stop codon TAA 1,290 bp downstream. DNA was prepared for microinjection by digesting the construct with XhoI and gel purifying the construct. Injections were performed into the pronuclear membrane of C57BL/6 zygotes at concentrations between 2 and 5 ng/μl, and the zygotes were transferred to pseudopregnant C57BL/6 females. Transgenic founders were mated with C57BL/6 mice, and heterozygous offspring were routinely identified by dot blots of genomic DNA (5 μg) and plasma assays (15 μl) for α-galactosidase activity.
RESULTS
Reduction of Erythrocyte Hemagglutination After Treatment with Purified α-Galactosidase.
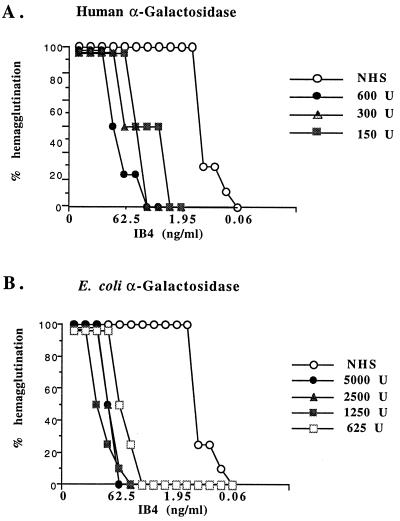
The ability of human α-galactosidase to cleave galactose from Galα(1,3)Gal was examined by using rabbit erythrocytes because the ceramide pentahexoside is the major Galα(1,3)Gal glycolipid of these cells (24). The concentration of the Galα(1,3)Gal-specific lectin IB4, from G. simplicifolia, required to cause agglutination of rabbit erythrocytes was used as an indication of antigen density before and after α-galactosidase treatment. The endpoint titer (50% hemagglutination) of IB4 lectin on untreated red cells was 0.98 ng/ml (Fig. 1). After treatment of the erythrocytes with either purified human or E. coli α-galactosidase, substantially more lectin was required to agglutinate the red cells: 7.8, 15.6, and 125 ng/ml of lectin after treatment with 150, 300, or 600 units of human α-galactosidase, respectively (Fig. 1A), and 62.5, 125, and 250 ng/ml of lectin after treatment with 625, 2500, or 5000 units of E. coli α-galactosidase (Fig. 1B). Thus, treatment of rabbit erythrocytes with α-galactosidase decreased the level of Galα(1,3)Gal on the cell surface (up to 255-fold using E. coli α-galactosidase). Clearly, human- or E. coli-derived α-galactosidase can be used to reduce the amount of antigen on erythrocytes, and our results are in agreement with earlier studies using coffee bean α-galactosidase (11–14) or bacteria-derived α-galactosidase (10) to remove α-galactosyl residues by enzyme treatment.
Figure 1.
Hemagglutination of erythrocytes after treatment with α-galactosidase. Direct hemagglutination assay showing the effect of treatment of rabbit erythrocytes with purified α-galactosidase. IB4 lectin at the indicated concentrations was incubated with untreated erythrocytes or cells treated with NHS or with (A) human α-galactosidase or (B) E. coli α-galactosidase at concentrations shown.
Reduction of Galα(1,3)Gal Produced by the Expression of α-Galactosidase cDNA.
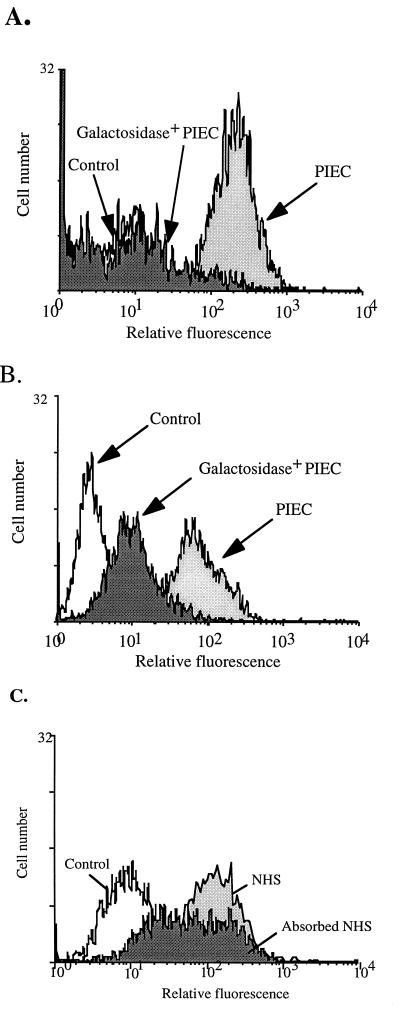
Clearly, α-galactosidase effectively can reduce the amount of Galα(1,3)Gal from the cell surface, and the enzyme could be used for the ex vivo treatment of xenograft donor organs; however, this method was cumbersome and would not address the problem of continual resynthesis of the epitope by α-galactosidase-treated cells. For xenotransplantation, a constant or constitutive expression of α-galactosidase in cells expressing the Gal epitope would be required to reduce the amount of Galα(1,3)Gal reaching the cell surface. To test this hypothesis PIEC cells were transfected with the human α-galactosidase cDNA in the expression vector pAsc9 or with vector alone, and five stable PIEC cell lines were generated and carried in vitro for several months. Flow cytometric analysis of cells that stably maintained the vector alone showed no significant difference in staining on the cell surface for Galα(1,3)Gal compared with nontransfected PIEC cells (Fig. 2A). In contrast, stable PIEC cell lines expressing human α-galactosidase showed up to a 30-fold reduction in the level of cell surface Galα(1,3)Gal (Fig. 2A). The reduction in the amount of Galα(1,3)Gal was inversely proportional both to the activity of α-galactosidase measured in cell lysates from separate clones and to the amount of cDNA transfected (data not shown).
Figure 2.
Binding of anti-Galα(1,3)Gal antibodies and human serum to α-galactosidase-transfected PIEC. FACS profiles of stable PIEC cell lines transfected with either human α-galactosidase cDNA or with vector alone were stained with (A) polyclonal chicken anti-laminin antibody to detect Galα(1,3)Gal and with FITC-conjugated goat anti-chicken IgG as control, (B) human natural IgG antibodies and with FITC-conjugated sheep anti-human Ig as control, or (C) NHS and NHS absorbed with Galα(1,3)Gal and with FITC-conjugated sheep anti-human Ig as control.
Cell lines also were tested for their ability to bind natural human anti-Galα(1,3)Gal antibodies, and cells expressing human α-galactosidase showed up to a 10-fold reduction in human IgG antibody binding compared with cells containing vector alone (Fig. 2B). To examine whether natural human antibodies would still bind to pig endothelial cells after removal of the Galα(1,3)Gal epitope, PIEC cells expressing human α-galactosidase were examined for their ability to bind normal human serum (NHS) that had been preabsorbed on a Galα(1,3)Gal column, i.e., all anti-Galα(1,3)Gal antibodies were removed (Fig. 2C). These cells still stained positive (Fig. 2C), demonstrating that PIEC have xenoepitopes other than Galα(1,3)Gal, including N-acetyl lactosamine (18), that are present and exposed when Galα(1,3)Gal is removed from the cell surface.
Transgenic Mice Expressing Human α-Galactosidase Have Decreased Natural Human Antibody Binding and Susceptibility to Human Antibody-Mediated Lysis.
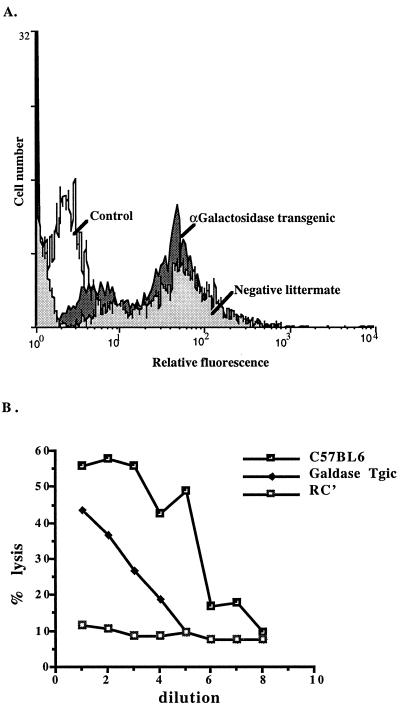
To determine whether constitutive expression of human α-galactosidase in vivo would result in decreased cell surface levels of Galα(1,3)Gal observed in vitro, as described above, transgenic mice expressing human α-galactosidase were generated. Splenocytes from three heterozygous α-galactosidase-transgenic mice (littermates) and from nontransgenic litter mates derived from one founder transgenic mouse were examined by flow cytometric analysis for their ability to bind natural human antibodies (Fig. 3A). FACS profiles of splenocytes showed two positive staining peaks: The brighter peak of splenocytes from transgenic mice had a mean channel fluorescence (mcf) of 48, and splenocytes from nontransgenic mice had a mcf of 58 (i.e., the transgenic mice showed a 15% reduction in binding to natural human antibodies). The duller peak on profiles of splenocytes from transgenic mice had a mcf of 6 compared with a mcf of 9 (i.e., the transgenic mice showed a 25% reduction in binding to natural human antibodies) (Fig. 3A). To assess the functional significance of this observed reduction, we used human antibody and complement-mediated lysis (Fig. 3B): 60% of splenocytes of normal mice were lysed by antibodies present in NHS with a 50% titer of 1/64. In contrast, the maximal lysis observed with transgenic splenocytes was 44%, with a 50% titer of 1/16. These results demonstrated that transgenic mice heterozygous for α-galactosidase had a small, but functionally significant, reduction in their ability to bind natural human anti-Galα(1,3)Gal antibodies and their susceptibility to human antibody-mediated lysis. The transgenic mice also demonstrated a decrease in their levels of cell surface Galα(1,3)Gal and an increase in α-galactosidase activity. Plasma enzyme levels in transgenic mice were at least triple those of nontransgenic littermates (78 ± 5 units/ml and 20 ± 2 units/ml, respectively). These results confirmed that constitutive in vivo expression of α-galactosidase is a feasible method of decreasing the Galα(1,3)Gal xenoepitope; presumably transgenic mice homozygous for α-galactosidase would further decrease the Galα(1,3)Gal epitope.
Figure 3.
Binding of human natural IgG antibodies and cytotoxicity of natural human antibodies on splenocytes from α-galactosidase transgenic mice. (A) FACS profiles of splenocytes from transgenic mice heterozygous for human α-galactosidase or nontransgenic littermates stained with human natural IgG antibodies. (B) Cytotoxicity of spleen cells from normal and α-galactosidase-transgenic mice. Vertical axis, % lysis; horizontal axis, doubling dilutions of human serum commencing at 1/2; RC’, rabbit complement. Results are representative of three experiments.
Coexpression of α-Galactosidase and α1,2-Fucosyltransferase Results in a Cumulative Decrease of Galα(1,3)Gal.
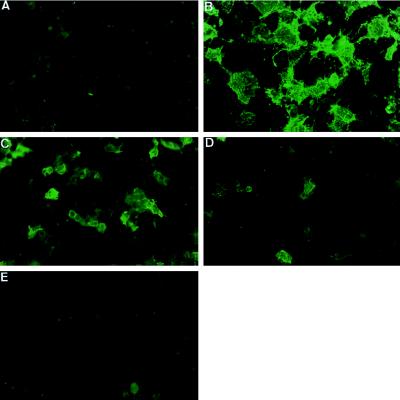
Although α-galactosidase removes Galα(1,3)Gal from the cell surface, it is clear that α-galactosidase alone, both in vitro and in vivo, will not result in the complete elimination of Galα(1,3)Gal. In addition, degalactosylation results in the exposure of N-acetyllactosamine residues and other potential new xenoepitopes (25). Previously, we reported that α1,2-fucosyltransferase can down-regulate Galα(1,3)Gal by ≈90% in vitro and in transgenic mice (18, 25). Therefore, we examined whether a combined approach using α-galactosidase and α1,2-fucosyltransferase was more effective than using either alone to remove Galα(1,3)Gal. Initially, this hypothesis was tested by transfection studies in COS cells, which previously established that cotransfection with both cDNAs for α1,3-galactosyltransferase and α1,2-fucosyltransferase resulted in the dominant expression of the H epitope and an almost complete absence of Galα(1,3)Gal (90% reduction) (18). Thus, the human α-galactosidase cDNA was cotransfected into COS cells with cDNAs for α1,3-galactosyltransferase and α1,2-fucosyltransferase and stained with IB4 lectin. Of cells expressing α1,3-galactosyltransferase alone, ≈60% of the cells stained with the IB4 lectin (Fig. 4B) and mock-transfected cells showed no IB4 staining (Fig. 4A). In cells cotransfected with cDNAs for α1,3-galactosyltransferase and α-galactosidase, ≈30% of the cells stained with IB4 (Fig. 4C) [i.e., a 50% reduction in Galα(1,3)Gal] whereas in cells coexpressing α1,3-galactosyltransferase plus α1,2-fucosyltransferase, there was only ≈10% of cells staining with IB4 (Fig. 4D), i.e., a 90% reduction in Galα(1,3)Gal. However, no IB4 staining was seen on cells cotransfected with all three cDNAs (Fig. 4E). Thus, cotransfection with α-galactosidase removed all of the residual Galα(1,3)Gal. Control transfections with plasmids containing α-galactosidase or α1,2-fucosyltransferase stained strongly with anti-α-galactosidase antibody or UEA1 (lectin specific for the H epitope), respectively, and they did not stain with IB4 (data not shown). In control transfections, ≈60% cells expressing α1,3-galactosyltransferase and CD48 stained with IB4 (data not shown), indicating that the observed reductions in IB4 staining with α-galactosidase and α1,2-fucosyltransferase reflect α-galactosidase and/or α1,2-fucosyltransferase activity alteration of the cell surface levels of Galα(1,3)Gal and not a result of the cotransfection procedure. These findings clearly demonstrate that α1,2-fucosyltransferase and α-galactosidase together have an additive effect in their ability to reduce the expression of Galα(1,3)Gal on the cell surface.
Figure 4.
Cell surface staining of transfected COS cells. IB4 lectin staining of cell surface of COS cells after: (A) mock transfection, (B) transfection with α1,3-galactosyltransferase cDNA, (C) transfection with α1,3-galactosyltransferase plus α-galactosidase cDNAs, (D) transfection with α1,3-galactosyltransferase plus α1,2-fucosyltransferase cDNAs, and (E) transfection with α1,3-galactosyltransferase plus α1,2-fucosyltransferase plus α-galactosidase cDNAs. Results are representative of at least 10 experiments.
Reduction in Complement-Mediated Cytotoxicity in Cells Transfected with α-Galactosidase and α1,2-Fucosyltransferase cDNAs.
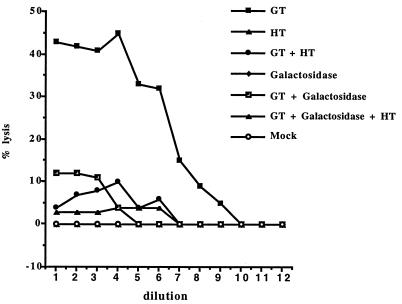
To assess the functional significance of this observed decrease in Galα(1,3)Gal expression, transfected COS cells were tested for their susceptibility to lysis by human serum and complement (Fig. 5). Forty-four percent of COS cells expressing Galα(1,3)Gal were lysed by antibodies in NHS and complement compared with a background lysis of 2%. There was a reduction in lysis to ≈10% of cells coexpressing α1,3-galactosyltransferase and α-galactosidase and ≈10% in cells coexpressing α1,3-galactosyltransferase and α1,2-fucosyltransferase. Lysis was reduced to background levels (2%) in cells expressing α-galactosidase and α1,2-fucosyltransferase. No lysis was observed in cells expressing α-galactosidase alone or α1,2-fucosyltransferase alone or in mock-transfected COS cells. The loss of susceptibility to lysis also was reflected in the serum titer: Cells expressing α1,3-galactosyltransferase alone showed a 50% lysis titer of 1/64; cells expressing α1,3-galactosyltransferase and α-galactosidase reduced this titer to 1/8, and for cells expressing α1,3-galactosyltransferase and α-galactosidase and α1,2-fucosyltransferase, the titer was 1/4. These results further confirm the findings that α1,2-fucosyltransferase and α-galactosidase have additive effects in eliminating Galα(1,3)Gal and reduce their susceptibility to human antibody-mediated lysis.
Figure 5.
Lysis of transfected COS cells by NHS. Pooled NHS was tested for lysis of transfected and nontransfected COS cells in a complement-mediated lysis assay. Titer of NHS on mock-transfected cells and cells transfected with α1,3-galactosyltransferase (GT), α-galactosidase (Galactosidase), and α1,2-fucosyltransferase (HT). Vertical axis, % lysis; horizontal axis, doubling dilutions of human serum commencing at 1/2. Results are representative of three experiments.
DISCUSSION
Because the major target of natural human antibodies in the hyperacute rejection of discordant xenotransplants is the antigen Galα(1,3)Gal (2, 3), strategies to prevent anti-Galα(1,3)Gal antibody reactivity are designed to remove the Galα(1,3)Gal antigen. In this study, we have shown that the constitutive transgenic expression of the human α-galactosyl cleaving enzyme α-galactosidase, in vitro and in vivo, can decrease the amount of Galα(1,3)Gal on the cell surface. Moreover, the Galα(1,3)Gal xenoepitope can be eliminated further in vitro by coexpressing α-galactosidase and α1,2-fucosyltransferase.
In vitro, human α-galactosidase was as efficient as E. coli-derived α-galactosidase to cleave Galα(1,3)Gal (Fig. 1); however, the low pH preference of the human enzyme [pH ≈5 (15)] would most likely prevent its use ex vivo on tissues or organs for any extended period of time without significant cell death. To date, the most useful enzyme for cleaving cell surface Galα(1,3)Gal has been coffee bean α-galactosidase, which works efficiently ex vivo at physiological pH to cleave galactosyl residues and convert group B to group O erythrocytes (26, 27). However, because the Galα(1,3)Gal epitope is continually resynthesized and replaced, efforts were directed to determine whether α-galactosidase could be constitutively expressed in cells, thereby decreasing the amount of cell surface Galα(1,3)Gal. Consistent with this concept, pig endothelial cell lines expressing human α-galactosidase showed up to a 30-fold reduction in the cell surface levels of Galα(1,3)Gal (Fig. 2). Although the Galα(1,3)Gal epitope was not eliminated in transfected COS cells that expressed α-galactosidase, the reduction of cell surface Galα(1,3)Gal was sufficient to markedly decrease the complement-mediated cytotoxicity (Fig. 5), thereby demonstrating that the reduction was functionally significant. That the constitutive expression of α-galactosidase can down-regulate surface Galα(1,3)Gal was further demonstrated in transgenic mice. In parallel with the results we described in vitro, α-galactosidase transgenics also demonstrated a decrease in the level of cell surface Galα(1,3)Gal, as well as a reduction in their binding to human natural antibodies (Fig. 3A) and reduced susceptibility to human antibody-mediated cell lysis (Fig. 3B). Our findings with the transgenic mice, although preliminary, clearly demonstrate that our transgenic approach using α-galactosidase to decrease Galα(1,3)Gal is viable and could be useful to overcome the replacement of the epitope by the continuous resynthesis of the Galα(1,3)Gal epitope. Future studies with homozygous transgenic mice will further clarify the merits of this strategy.
In reducing the cell surface Galα(1,3)Gal by constitutive expression of α-galactosidase, subterminal saccharides are exposed (i.e., N-acetyl lactosamine), resulting in the development of antibody reactions to the newly unmasked epitopes. Indeed, human serum depleted of antibodies to Galα(1,3)Gal still bound to PIEC cells expressing α-galactosidase (Fig. 2), confirming that other xenoepitopes become exposed on the surface of these cells. These studies were not designed to determine the site of α-galactosidase action that reduced the cell surface Galα(1,3)Gal. An intracellular site is likely because the enzyme has an acid pH optimum (pH 4.5) (28). It is more likely that the overexpressed α-galactosidase accumulated in the trans Golgi, shown to occur in Chinese hamster ovary cells overexpressing human α-galactosidase (15), where the estimated pH of ≈6 would be more conducive to the cleavage of terminal galactosyl residues from oligosaccharides, resulting in the decreased levels of Galα(1,3)Gal observed in this study. Further characterization of the α-galactosidase transgenic mice may provide insight into the mechanisms involved in this process. These findings are in agreement with studies that used coffee bean α-galactosidase in culture to remove Galα(1,3)Gal from the surface of porcine aortic endothelial cells and demonstrated the exposure of cryptic- or neo-antigens, including N-acetyl lactosamine (14). Previously, we reported that the surface Galα(1,3)Gal on cells could be reduced up to 90% by the intracellular competition between α1,3-galactosyltransferase and α1,2-fucosyltransferase, resulting in the substitution of Galα(1,3)Gal with the nonimmunogenic H substance (18, 29). The overexpression of α-galactosidase results in the exposure of N-acetyl lactosamine, so a logical step was to test whether α-galactosidase and α1,2-fucosyltransferase could be used together to efficiently remove Galα(1,3)Gal and concurrently cap the exposed saccharides with a terminal fucose residue. Clearly, there was a greater reduction in surface Galα(1,3)Gal achieved by using a double transfection strategy with α-galactosidase and α1,2-fucosyltransferase than with either enzyme alone. IB4 staining was not detected on COS cells expressing both α-galactosidase and α1,2-fucosyltransferase (Fig. 4) whereas IB4 staining of cells expressing only α-galactosidase was reduced by ≈50% and of cells expressing only α1,2-fucosyltransferase was decreased ≈90% (Fig. 4). The additive effect of these two enzymes was reflected in the susceptibility of these cells to complement-mediated lysis in the presence of NHS with a maximal reduction in lysis to background levels when both enzymes were expressed in transfected cells (Fig. 5).
Thus, these in vitro results demonstrate that Galα(1,3)Gal can be eliminated from the cell surface more effectively by using α-galactosidase in combination with α1,2-fucosyltransferase than by either enzyme alone. Although gene knockouts currently are not technically feasible in the pig, transgenic pigs can be generated and bred. This study suggests that a combined transgenic approach using α-galactosidase and α1,2-fucosyltransferase will result in the continuous suppression of Galα(1,3)Gal on the cell surface of donor tissues, thereby providing a powerful tool for the production of phenotypically Galα(1,3)Gal-negative pig tissues for xenotransplantation.
Acknowledgments
This work was supported in part by funds from the National Health and Medical Research Council of Australia.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: FITC, fluorescein isothiocyanate; mcf, mean channel fluorescence; NHS, normal human serum.
References
- 1.Sandrin M S, Vaughan H A, McKenzie I F C. Transplant Rev. 1994;8:134–149. [Google Scholar]
- 2.Cooper D K C, Koren E, Oriol R. Immunol Rev. 1994;141:31–58. doi: 10.1111/j.1600-065x.1994.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandrin M S, McKenzie I F C. Immunol Rev. 1994;141:169–190. doi: 10.1111/j.1600-065x.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandrin M S, Cohney S, Osman N, McKenzie I F C. In: Xenotransplantation. Cooper D K C, Kemp E, Platt J L, White D J G, editors. Berlin: Springer; 1997. pp. 683–700. [Google Scholar]
- 5.McCurry K R, Kooyman D L, Alvarado C G, Cotterell A H, Martin M J, Logan J S, Platt J L. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 6.Oriol R, Ye Y, Koren E, Cooper D K. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Strahan K M, Preece A F, Xu Y, Gustafsson K. Xenotransplantation. 1995;2:143–147. [Google Scholar]
- 8.Sandrin M S, Dabkowski P L, Henning M M, Mouhtouris E, McKenzie I F C. Xenotransplantation. 1994;1:81–88. [Google Scholar]
- 9.Capecchi M R. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 10.Cairns T, Hammelmann D, Gray K, Welsh K, Larson G. Transplant Proc. 1994;26:1279–1280. [PubMed] [Google Scholar]
- 11.Collins B H, Parker W, Platt J L. Xenotransplantation. 1994;1:36–46. [Google Scholar]
- 12.Holznecht Z E, Platt J L. J Immunol. 1995;154:4565–4575. [PubMed] [Google Scholar]
- 13.Lavecchio J A, Dunne A D, Edge A S B. Transplantation. 1995;60:841–847. [PubMed] [Google Scholar]
- 14.Watier H, Guillaumin J-M, Piller F, Lacord M, Thibault G, Lebranchu Y, Monsigny M, Bardos P. Transplantation. 1996;62:105–113. doi: 10.1097/00007890-199607150-00020. [DOI] [PubMed] [Google Scholar]
- 15.Ioannou Y A, Bishop D F, Desnick R J. J Cell Biol. 1992;119:1137–1150. doi: 10.1083/jcb.119.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes C E, Goldstein I J. J Biol Chem. 1974;6:1904–1914. [PubMed] [Google Scholar]
- 17.Vaughan H A, Henning M M, Purcell D F J, McKenzie I F C, Sandrin M S. Immunogenetics. 1991;33:113–117. doi: 10.1007/BF00210824. [DOI] [PubMed] [Google Scholar]
- 18.Sandrin M S, Fodor W F, Mouhtouris E, Osman N, Cohney S C, Rollins S A, Guilmette E R, Setter E, Squinto S P, McKenzie I F C. Nat Med. 1995;1:1261–1267. doi: 10.1038/nm1295-1261. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel F M. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 20.Kint J A. Science. 1970;270:1268–1269. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- 21.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan H A, Loveland B E, Sandrin M S. Transplantation. 1994;58:879–882. doi: 10.1097/00007890-199410270-00003. [DOI] [PubMed] [Google Scholar]
- 23.Weiss E, Golden L, Zakut R, Mellor A, Fahrner K, Kvist S, Flavell R A. EMBO J. 1983;2:453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galili U, Shohet S B, Korbin E, Stults C L M, Macher B A. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 25.Cohney S, Mouhtouris E, McKenzie I F C, Sandrin M S. Immunogenetics. 1996;44:76–79. doi: 10.1007/BF02602660. [DOI] [PubMed] [Google Scholar]
- 26.Lenny L L, Hurst R, Goldstein J, Benjamin L J, Jones R L. Blood. 1991;77:1383–1388. [PubMed] [Google Scholar]
- 27.Lenny L L, Hurst R, Zhu A, Goldstein J, Galbraith R A. Transfusion. 1995;35:899–902. doi: 10.1046/j.1537-2995.1995.351196110892.x. [DOI] [PubMed] [Google Scholar]
- 28.Flowers H M, Sharon N. Adv Enzymol Relat Areas Mol Biol. 1979;48:29–95. doi: 10.1002/9780470122938.ch2. [DOI] [PubMed] [Google Scholar]
- 29.Cohney S, McKenzie I F C, Patton K, Prenzoska J, Ostenreid K, Fodor W L, Sandrin M S. Transplantation. 1997;64:495–500. doi: 10.1097/00007890-199708150-00020. [DOI] [PubMed] [Google Scholar]