Abstract
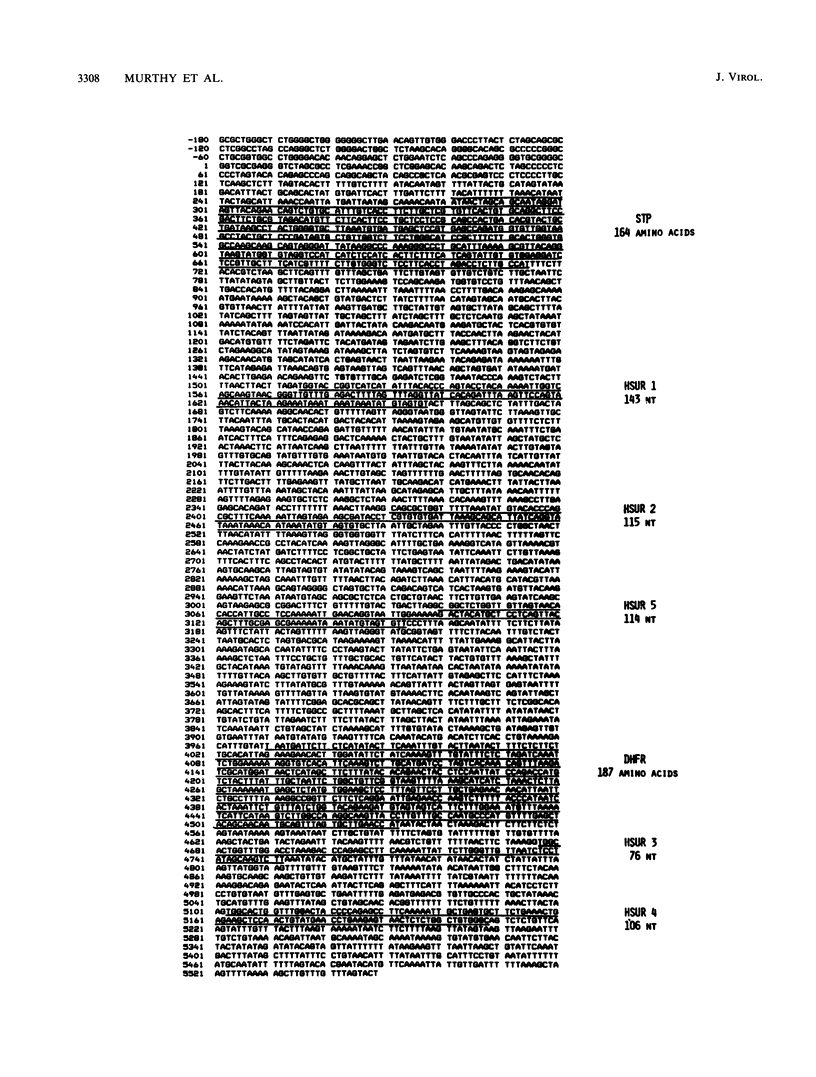
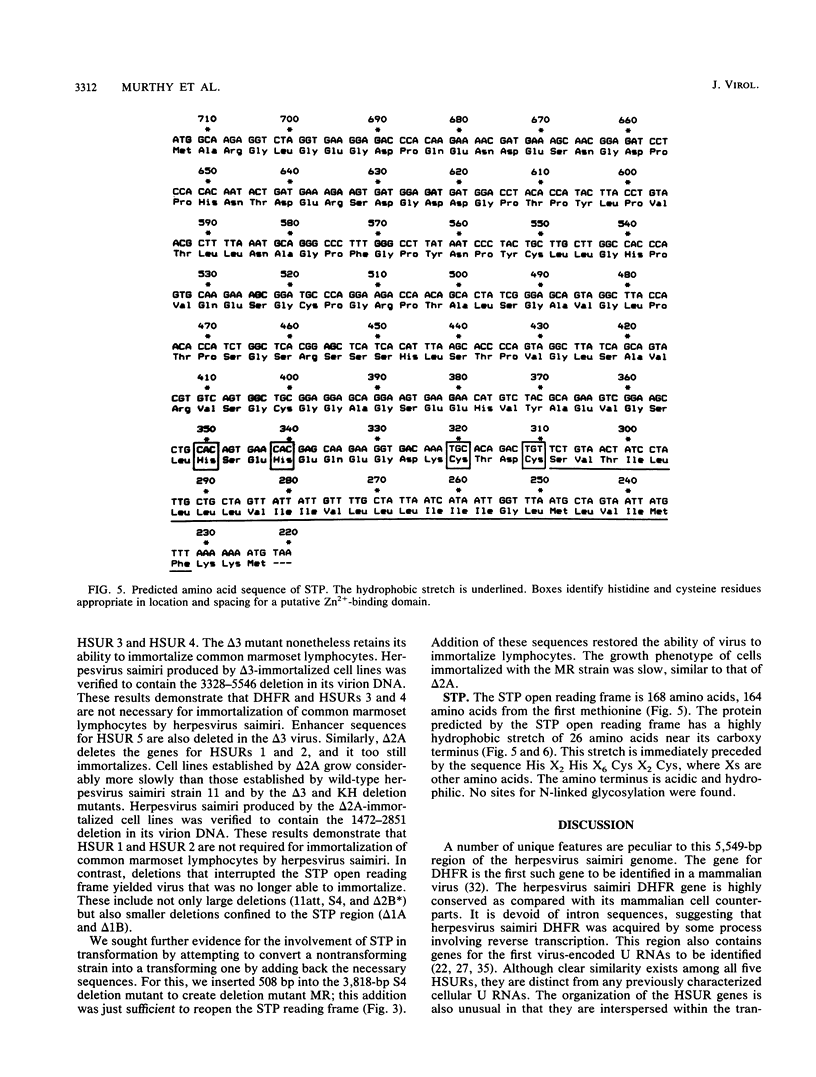
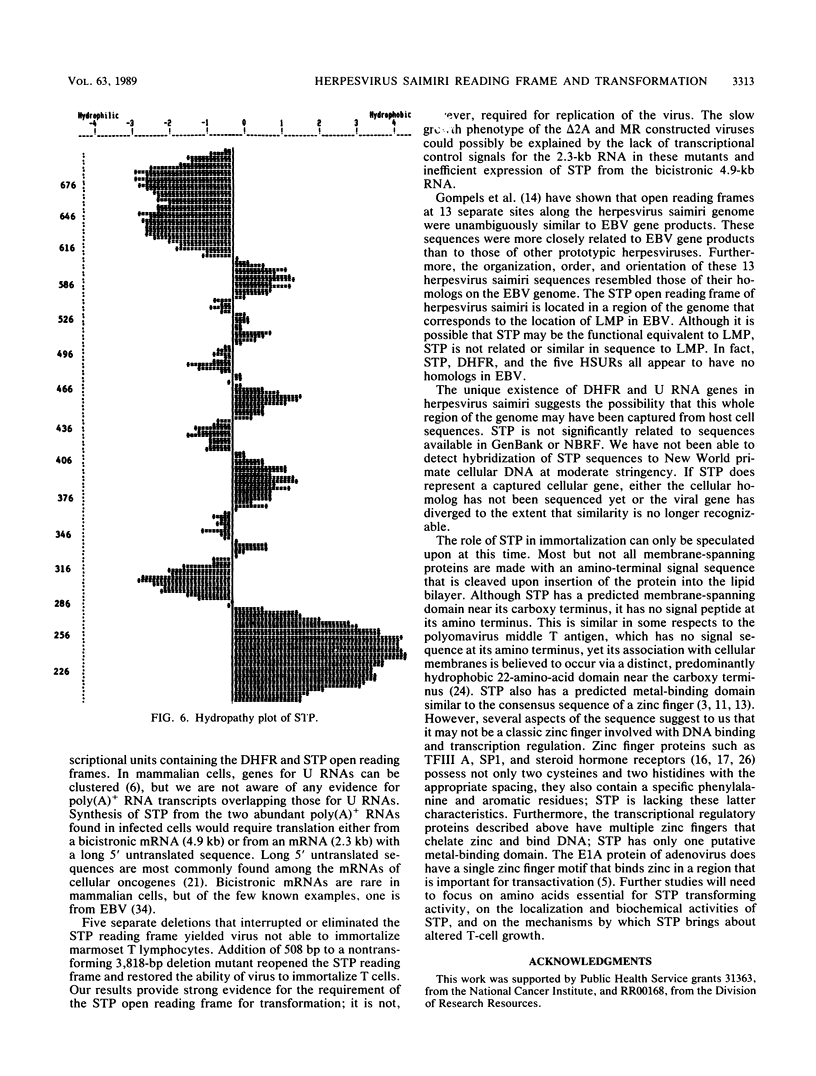
Analysis of a 5,549-base-pair sequence at the left end of herpesvirus saimiri unique (L-) DNA revealed two open reading frames and genes for five small nuclear U RNAs (herpesvirus saimiri U RNAs). Replication-competent deletion mutants were constructed in order to assess the importance of these genetic features for transformation by this oncogenic herpesvirus. Although not required for replication, one of the open reading frames was found to be required for immortalization of marmoset T lymphocytes into continuously growing cell lines. The protein predicted by this reading frame (STP; saimiri transformation-associated protein) has a highly hydrophobic stretch of 26 amino acids sufficient for a membrane-spanning domain near its carboxy terminus; this domain is immediately preceded by a sequence appropriate for formation of a metal-binding domain (His X2 His X6 Cys X2 Cys, where Xs are other amino acids). One of two poly(A)+ RNAs that could encode STP is bicistronic, while the other has a long 5' untranslated region (approximately 1.5 kilobases). Although some analogies can be drawn between STP and LMP (lymphocyte membrane protein) of Epstein-Barr virus, STP is not related in sequence to LMP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankier A. T., Dietrich W., Baer R., Barrell B. G., Colbère-Garapin F., Fleckenstein B., Bodemer W. Terminal repetitive sequences in herpesvirus saimiri virion DNA. J Virol. 1985 Jul;55(1):133–139. doi: 10.1128/jvi.55.1.133-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp J. S., Webster L. C., Friedman D. J., Smith C. L., Huang W. J., Wu F. Y., Rosenberg M., Ricciardi R. P. The 289-amino acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6450–6454. doi: 10.1073/pnas.85.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
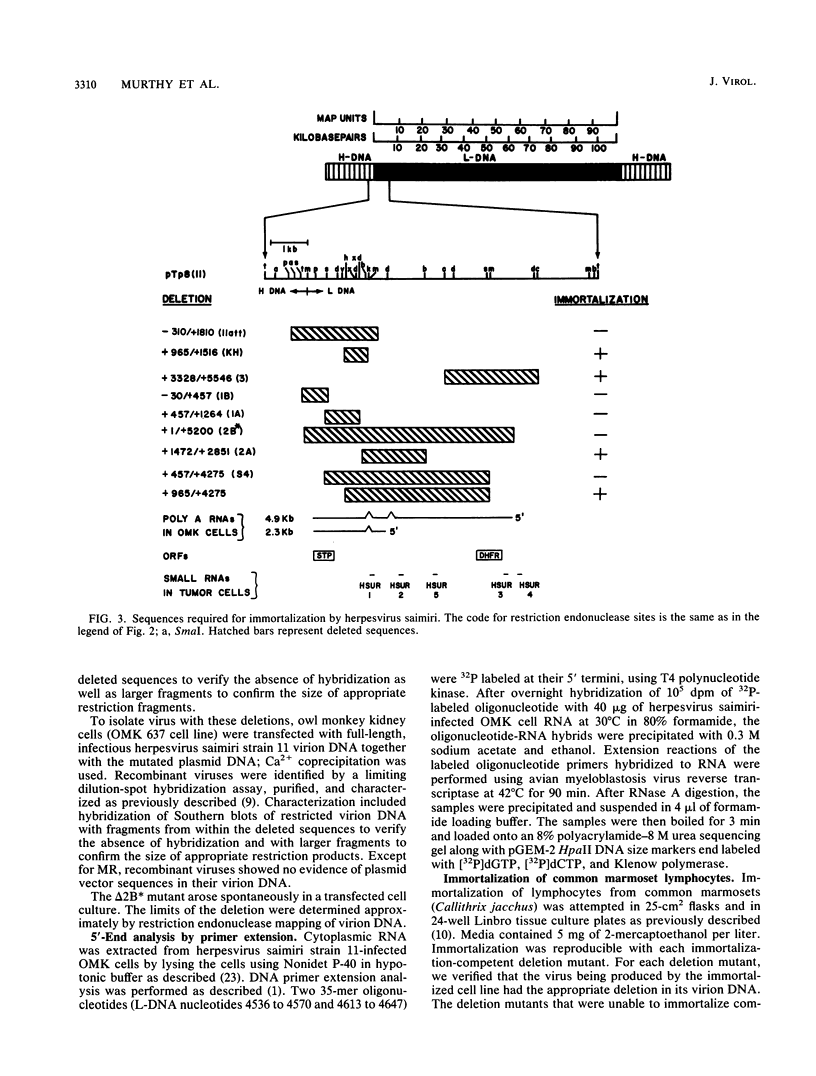
- Desrosiers R. C., Bakker A., Kamine J., Falk L. A., Hunt R. D., King N. W. A region of the Herpesvirus saimiri genome required for oncogenicity. Science. 1985 Apr 12;228(4696):184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Burghoff R. L., Bakker A., Kamine J. Construction of replication-competent Herpesvirus saimiri deletion mutants. J Virol. 1984 Feb;49(2):343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Silva D. P., Waldron L. M., Letvin N. L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986 Feb;57(2):701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Fingering too many proteins. Cell. 1988 Jun 3;53(5):675–675. doi: 10.1016/0092-8674(88)90083-9. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of gene organization in the lymphotropic herpesviruses herpesvirus Saimiri and Epstein-Barr virus. J Virol. 1988 Mar;62(3):757–767. doi: 10.1128/jvi.62.3.757-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kamine J., Bakker A., Desrosiers R. C. Mapping of RNA transcribed from a region of the Herpesvirus saimiri genome required for oncogenicity. J Virol. 1984 Nov;52(2):532–540. doi: 10.1128/jvi.52.2.532-540.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
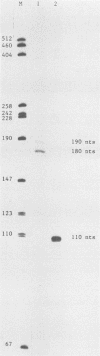
- Lee S. I., Murthy S. C., Trimble J. J., Desrosiers R. C., Steitz J. A. Four novel U RNAs are encoded by a herpesvirus. Cell. 1988 Aug 26;54(5):599–607. doi: 10.1016/s0092-8674(88)80004-7. [DOI] [PubMed] [Google Scholar]
- Markland W., Cheng S. H., Oostra B. A., Smith A. E. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J Virol. 1986 Jul;59(1):82–89. doi: 10.1128/jvi.59.1.82-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez L. V., Daniel M. D., Hunt R. D., Garcia F. G. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab Anim Care. 1968 Jun;18(3):374–381. [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S., Kamine J., Desrosiers R. C. Viral-encoded small RNAs in herpes virus saimiri induced tumors. EMBO J. 1986 Jul;5(7):1625–1632. doi: 10.1002/j.1460-2075.1986.tb04405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer R. H., Briggs C. J., Noer K. B., Rabin H. Identification of normal and transformed lymphocyte subsets of nonhuman primates with monoclonal antibodies to human lymphocytes. J Immunol. 1983 Mar;130(3):1323–1329. [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Falk L. A., Deinhardt F. Attenuation of herpesvirus saimiri for marmosets after successive passage in cell culture at 39 degrees C. J Natl Cancer Inst. 1975 Nov;55(5):1243–1246. doi: 10.1093/jnci/55.5.1243. [DOI] [PubMed] [Google Scholar]
- Trimble J. J., Murthy S. C., Bakker A., Grassmann R., Desrosiers R. C. A gene for dihydrofolate reductase in a herpesvirus. Science. 1988 Mar 4;239(4844):1145–1147. doi: 10.1126/science.2830673. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman D. A., Lee S. I., Steitz J. A. Nucleotide sequence of HSUR 5 RNA from herpesvirus saimiri. Nucleic Acids Res. 1989 Feb 11;17(3):1258–1258. doi: 10.1093/nar/17.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Falk L. A., Collins D., Deinhardt F. Mononuclear cell fraction carrying Herpesvirus saimiri in persistently infected squirrel monkeys. J Natl Cancer Inst. 1976 Oct;57(4):959–962. doi: 10.1093/jnci/57.4.959. [DOI] [PubMed] [Google Scholar]