Abstract
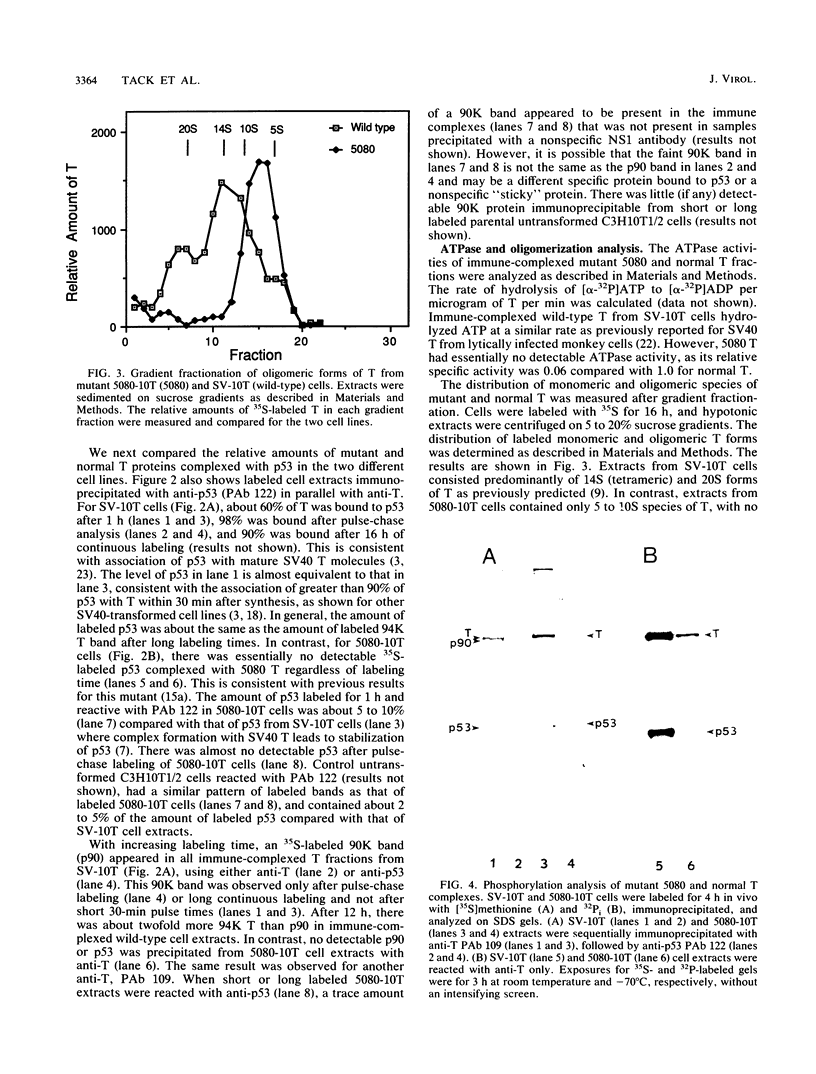
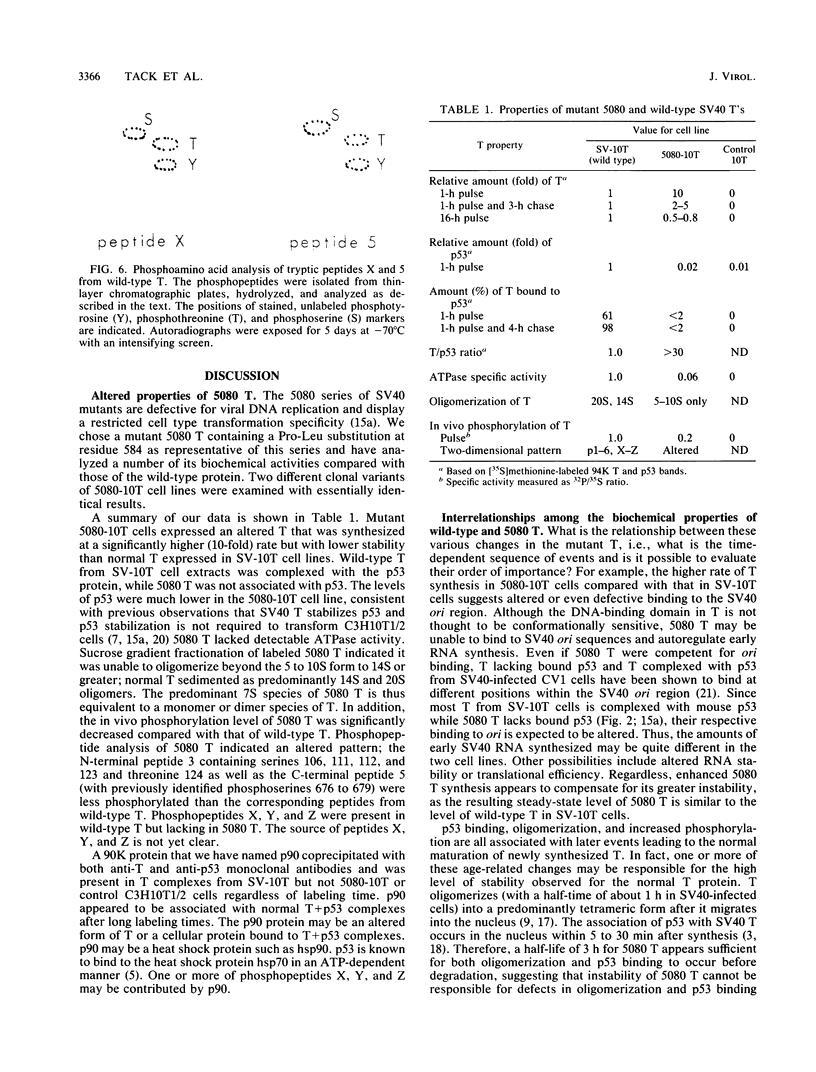
We have analyzed the biochemical properties of a nonviable simian virus 40 (SV40) mutant encoding a large T antigen (T) bearing an amino acid substitution (Pro-584-Leu) in its hydrophobic region. Mutant 5080 has an altered cell type specificity for transformation (transforming mouse C3H10T1/2 but not rat REF52 cells), is defective for viral DNA replication, and encodes a T that is unable to form a complex with the cellular p53 protein (K. Peden, A. Srinivasan, J. Farber, and J. Pipas, Virology 168:13-21, 1989). In this article, we show that 5080-transformed C3H10T1/2 cell lines express an altered T that is synthesized at a significantly higher rate but with a shorter half-life than normal T from wild-type SV40-transformed cells. 5080 T did not oligomerize beyond 5 to 10S in size compared with normal T, which oligomerized predominantly to 14 to 20S species. In addition, the 5080 T complex had significantly decreased ATPase activity and had a 10-fold-lower level of in vivo phosphorylation compared with that of normal T. Two-dimensional phosphopeptide analysis indicated several changes in the specific 32P labeling pattern, with altered phosphorylation occurring at both termini of the mutant protein compared with the wild-type T. Loss of p53 binding is therefore concomitant with changes in ATPase activity, oligomerization, stability, and in vivo phosphorylation of T and can be correlated with defective replication and restricted transformation functions. That so many biochemical changes are associated with a single substitution in the hydrophobic region of T is consistent with its importance in regulating higher-order structural and functional relationships in SV40 T.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Gurney E. G. Time-dependent maturation of the simian virus 40 large T antigen-p53 complex studied by using monoclonal antibodies. J Virol. 1982 Nov;44(2):565–573. doi: 10.1128/jvi.44.2.565-573.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. F., Cheng K., Frey A. B., Stein R., Hinds P. W., Levine A. J. Purification of complexes of nuclear oncogene p53 with rat and Escherichia coli heat shock proteins: in vitro dissociation of hsc70 and dnaK from murine p53 by ATP. Mol Cell Biol. 1988 Mar;8(3):1206–1215. doi: 10.1128/mcb.8.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M. The p53 cellular tumor antigen: gene structure, expression and protein properties. Biochim Biophys Acta. 1985 Nov 12;823(1):67–78. doi: 10.1016/0304-419x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Srinivasan A., Farber J. M., Pipas J. M. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology. 1989 Jan;168(1):13–21. doi: 10.1016/0042-6822(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickedanz J., Scheidtmann K. H., Walter G. Kinetics of nuclear transport and oligomerization of simian virus 40 large T antigen. Virology. 1986 Jan 15;148(1):47–57. doi: 10.1016/0042-6822(86)90402-2. [DOI] [PubMed] [Google Scholar]
- Schmieg F. I., Simmons D. T. Intracellular location and kinetics of complex formation between simian virus 40 T antigen and cellular protein p53. J Virol. 1984 Nov;52(2):350–355. doi: 10.1128/jvi.52.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann C., Montenarh M., Kohler M., Henning R. Oligomerization of simian virus 40 tumor antigen may be involved in viral DNA replication. Virology. 1985 Oct 15;146(1):1–11. doi: 10.1016/0042-6822(85)90047-9. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Gurney E. G., Danna K. J. Stabilization of the 53,000-dalton nonviral tumor antigen is not required for transformation by simian virus 40. Mol Cell Biol. 1983 Feb;3(2):290–296. doi: 10.1128/mcb.3.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Deb S. P., Tegtmeyer P. The p53 complex from monkey cells modulates the biochemical activities of simian virus 40 large T antigen. J Virol. 1989 Mar;63(3):1310–1317. doi: 10.1128/jvi.63.3.1310-1317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Alterations in the structure of new and old forms of simian virus 40 large T antigen (T) defined by age-dependent epitope changes: new T is the same as ATPase-active T. J Virol. 1989 May;63(5):2352–2356. doi: 10.1128/jvi.63.5.2352-2356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Characterization of simian virus 40 large T antigen by using different monoclonal antibodies: T-p53 complexes are preferentially ATPase active and adenylylated. J Virol. 1988 Mar;62(3):1028–1037. doi: 10.1128/jvi.62.3.1028-1037.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]