Abstract
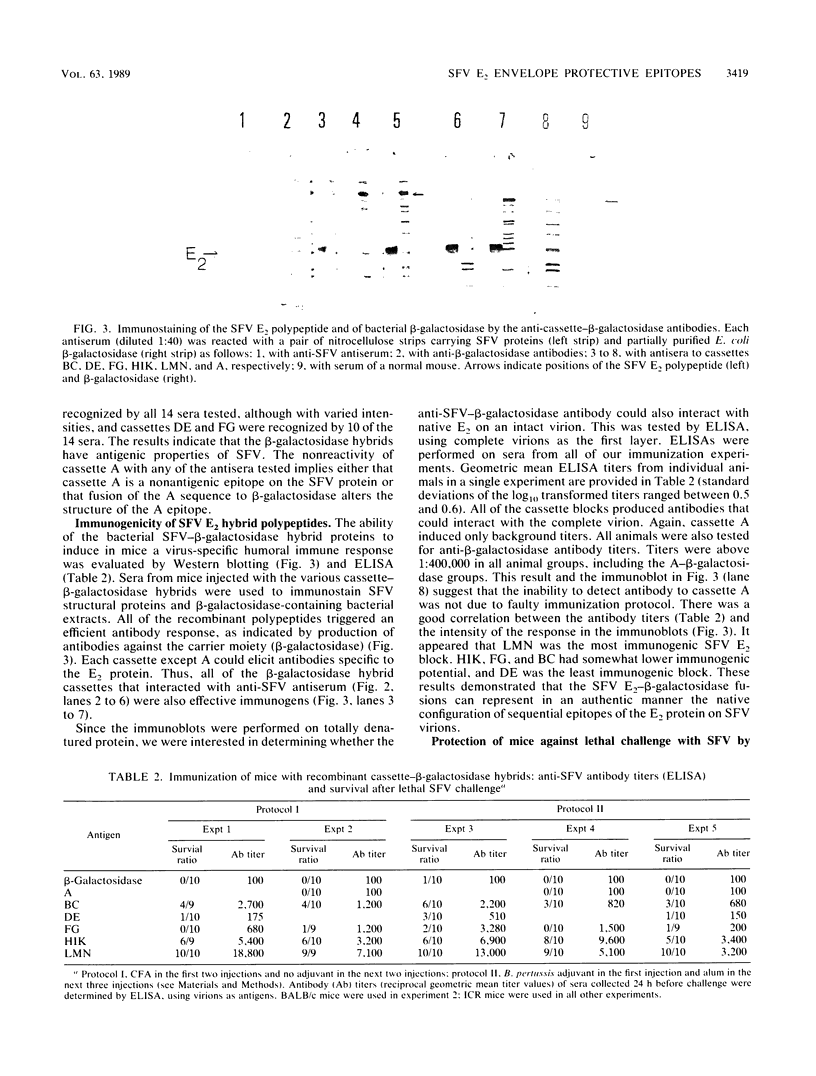
Along the 422 amino acids of the Semliki Forest virus (SFV) E2 envelope glycoprotein, we identified 13 peptide cassettes (ranging in size from 15 to 25 amino acids and designated A through N) that contain hydrophilic sequences flanked by amino acid sequences conserved in the E2 envelopes of the alphavirus family. Six peptide blocks containing either a single cassette or two to three contiguous cassettes (A, BC, DE, FG, HIK, and LMN) were produced in Escherichia coli as recombinant proteins fused to the N terminus of beta-galactosidase. All of the SFV E2 recombinant polypeptides except A-beta-galactosidase were recognized on Western blots (immunoblots) by anti-SFV polyclonal antisera. In addition, these five recombinant proteins induced in mice antibodies that interacted specifically with SFV E2 protein on Western blots as well as with the intact virions in an enzyme-linked immunosorbent assay. The six hybrid proteins were used to vaccinate mice and were tested for the ability to confer resistance against lethal doses of SFV. Peptides BC and HIK, located at amino acid positions 114 to 149 and 216 to 288, respectively, of E2, protected partially (40 to 60%) against SFV challenge. A third peptide, LMN, located between amino acid positions 289 and 352, rendered mice totally resistant to an SFV challenge of 250 50% lethal doses. The partially protective effects of the BC and HIK cassettes and the high efficacy of the LMN cassette were consistently demonstrated, independent of the adjuvant (complete Freund or alum), immunization protocol, and strain of mice used. None of the antisera raised against any given cassette could neutralize the virus in an in vitro tissue culture assay or in a plaque reduction neutralization test. Nevertheless, passive transfer experiments demonstrated that in the case of LMN, the protective effect was mainly of a humoral nature.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisiegel U., Schneider W. J., Brown M. S., Goldstein J. L. Immunoblot analysis of low density lipoprotein receptors in fibroblasts from subjects with familial hypercholesterolemia. J Biol Chem. 1982 Nov 10;257(21):13150–13156. [PubMed] [Google Scholar]
- Bell J. R., Kinney R. M., Trent D. W., Strauss E. G., Strauss J. H. An evolutionary tree relating eight alphaviruses, based on amino-terminal sequences of their glycoproteins. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4702–4706. doi: 10.1073/pnas.81.15.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. The role of complement in monoclonal antibody-mediated protection against virulent Semliki Forest virus. Immunology. 1986 Aug;58(4):553–559. [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. W., Schlesinger J. J., Walsh E. E., Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986 Feb;67(Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Shope R. E., Brandt W., Casals J., Karabatsos N., Murphy F. A., Tesh R. B., Wiebe M. E. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology. 1980;14(5-6):229–232. doi: 10.1159/000149190. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Kondor-Koch C., Riedel H., Söderberg K., Garoff H. Expression of the structural proteins of Semliki Forest virus from cloned cDNA microinjected into the nucleus of baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4525–4529. doi: 10.1073/pnas.79.15.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig J. T., Gorski D., Schlesinger M. J. Properties of monoclonal antibodies directed against the glycoproteins of Sindbis virus. J Gen Virol. 1982 Apr;59(Pt 2):421–425. doi: 10.1099/0022-1317-59-2-421. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]