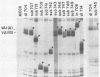
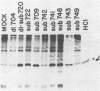
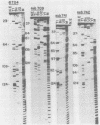
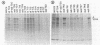Abstract
During the course of adenovirus infection, the VAI RNA protects the translation apparatus of host cells by preventing the activation of host double-stranded RNA-activated protein kinase, which phosphorylates and thereby inactivates the protein synthesis initiation factor eIF-2. In the absence of VAI RNA, protein synthesis is drastically inhibited at late times in infected cells. The experimentally derived secondary structure of VAI RNA consists of two extended base-paired regions, stems I and III, which are joined by a short base-paired region, stem II, at the center. Stems I and II are joined by a small loop, A, and stem III contains a hairpin loop, B. At the center of the molecule and at the 3' side, stems II and III are connected by a short stem-loop (stem IV and hairpin loop C). A fourth, minor loop, D, exists between stems II and IV. To determine sequences and domains critical for function within this VAI RNA structure, we have constructed adenovirus mutants with linker-scan substitution mutations in defined regions of the molecule. Cells infected with these mutants were analyzed for polypeptide synthesis, virus yield, and eIF-2 alpha kinase activity. Our results showed that disruption of base-paired regions in the distal parts of the longest stems, I and III, did not affect function, whereas mutations causing structural perturbations in the central part of the molecule containing stem II, the proximal part of stem III, and the central short stem-loop led to loss of function. Surprisingly, one substitution mutant, sub742, although dramatically perturbing the integrity of the structure of this central portion, showed a wild-type phenotype, suggesting that an RNA with an alternate secondary structure is functional. On the basis of sensitivity to single-strand-specific RNases, we can derive a novel secondary structure for the mutant RNA in which a portion of the sequences may fold to form a structure that resembles the central part of the wild-type molecule, which suggests that only the short stem-loop located in the center of the molecule and the adjoining base-paired regions may define the functional domain. These results also imply that only a portion of the VAI RNA structure may be recognized by the host factor(s).
Full text
PDF


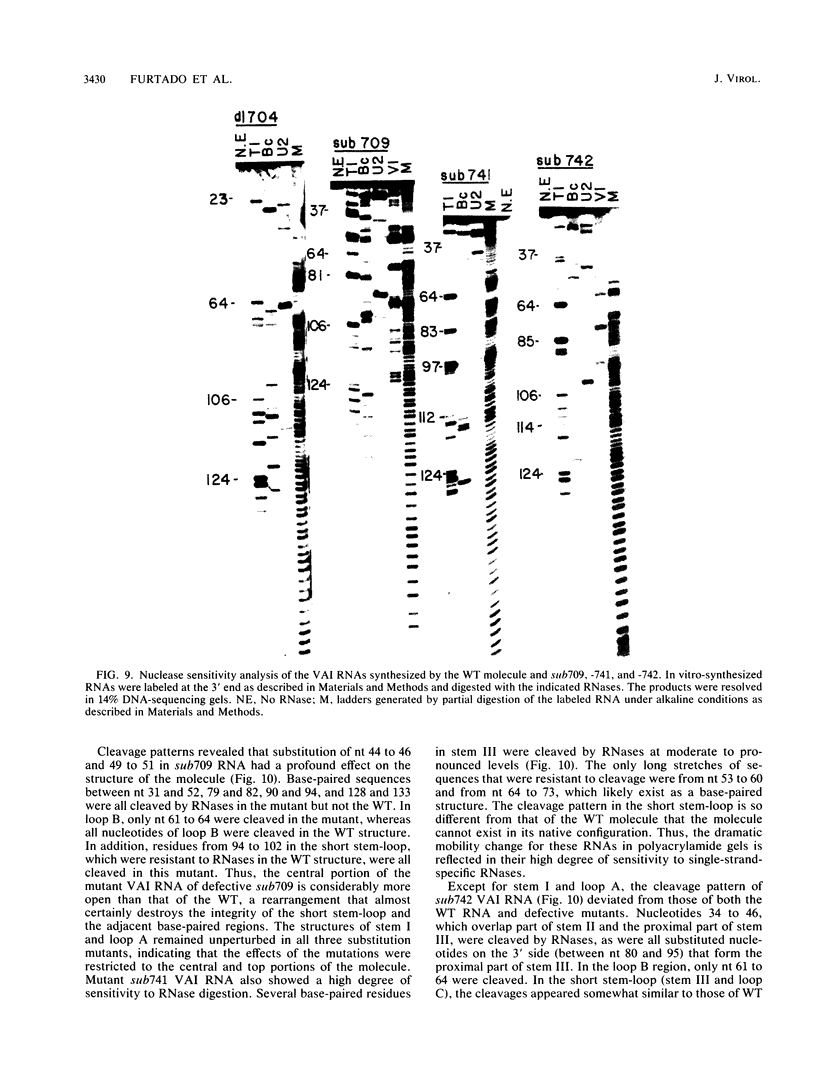
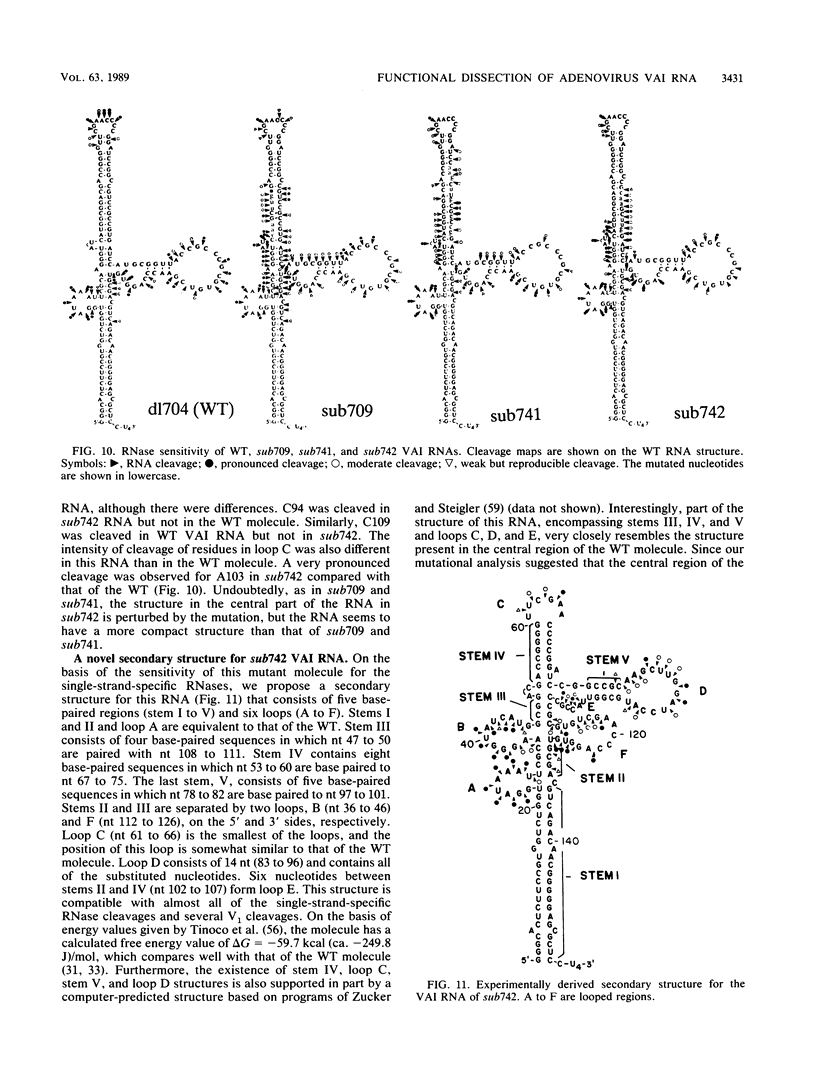



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akusjärvi G., Svensson C., Nygård O. A mechanism by which adenovirus virus-associated RNAI controls translation in a transient expression assay. Mol Cell Biol. 1987 Jan;7(1):549–551. doi: 10.1128/mcb.7.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Knutson G. S., Lasky S. R., Munemitsu S. M., Samuel C. E. Mechanism of interferon action. Purification and substrate specificities of the double-stranded RNA-dependent protein kinase from untreated and interferon-treated mouse fibroblasts. J Biol Chem. 1985 Sep 15;260(20):11240–11247. [PubMed] [Google Scholar]
- Bhat R. A., Domer P. H., Thimmappaya B. Structural requirements of adenovirus VAI RNA for its translation enhancement function. Mol Cell Biol. 1985 Jan;5(1):187–196. doi: 10.1128/mcb.5.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Metz B., Thimmappaya B. Organization of the noncontiguous promoter components of adenovirus VAI RNA gene is strikingly similar to that of eucaryotic tRNA genes. Mol Cell Biol. 1983 Nov;3(11):1996–2005. doi: 10.1128/mcb.3.11.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Adenovirus mutants with DNA sequence perturbations in the intragenic promoter of VAI RNA gene allow the enhanced transcription of VAII RNA gene in HeLa cells. Nucleic Acids Res. 1984 Oct 11;12(19):7377–7388. doi: 10.1093/nar/12.19.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Construction and analysis of additional adenovirus substitution mutants confirm the complementation of VAI RNA function by two small RNAs encoded by Epstein-Barr virus. J Virol. 1985 Dec;56(3):750–756. doi: 10.1128/jvi.56.3.750-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. I. The sequences at the 3' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9032–9042. [PubMed] [Google Scholar]
- Cepko C. L., Sharp P. A. Analysis of Ad5 hexon and 100K ts mutants using conformation-specific monoclonal antibodies. Virology. 1983 Aug;129(1):137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- Engler J. A., Hoppe M. S., van Bree M. P. The nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 7. Gene. 1983 Jan-Feb;21(1-2):145–159. doi: 10.1016/0378-1119(83)90156-7. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Guilfoyle R., Weinmann R. Control region for adenovirus VA RNA transcription. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3378–3382. doi: 10.1073/pnas.78.6.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Steitz J. A. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Kimchi A., Zilberstein A., Schmidt A., Shulman L., Revel M. The interferon-induced protein kinase PK-i from mouse L cells. J Biol Chem. 1979 Oct 10;254(19):9846–9853. [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Shenk T. An adenovirus mutant unable to express VAI RNA displays different growth responses and sensitivity to interferon in various host cell lines. Mol Cell Biol. 1986 Dec;6(12):4493–4498. doi: 10.1128/mcb.6.12.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Larsson S., Bellett A., Akusjärvi G. VA RNAs from avian and human adenoviruses: dramatic differences in length, sequence, and gene location. J Virol. 1986 May;58(2):600–609. doi: 10.1128/jvi.58.2.600-609.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S., Svensson C., Akusjärvi G. Characterization of a low-molecular-weight virus-associated (VA) RNA encoded by simian adenovirus type 7 which functionally can substitute for adenovirus type 5 VA RNAI. J Virol. 1986 Nov;60(2):635–644. doi: 10.1128/jvi.60.2.635-644.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Maran A., Mathews M. B. Characterization of the double-stranded RNA implicated in the inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA. Virology. 1988 May;164(1):106–113. doi: 10.1016/0042-6822(88)90625-3. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Genes for VA-RNA in adenovirus 2. Cell. 1975 Oct;6(2):223–229. doi: 10.1016/0092-8674(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Grodzicker T. Virus-associated RNAs of naturally occurring strains and variants of group C adenoviruses. J Virol. 1981 Jun;38(3):849–862. doi: 10.1128/jvi.38.3.849-862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mellits K. H., Mathews M. B. Effects of mutations in stem and loop regions on the structure and function of adenovirus VA RNAI. EMBO J. 1988 Sep;7(9):2849–2859. doi: 10.1002/j.1460-2075.1988.tb03141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., West D. K., Benvin S., Baglioni C. Structural requirements of double-stranded RNA for the activation of 2',5'-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells. J Biol Chem. 1979 Oct 25;254(20):10180–10183. [PubMed] [Google Scholar]
- Monstein H. J., Philipson L. The conformation of adenovirus VAI-RNA in solution. Nucleic Acids Res. 1981 Sep 11;9(17):4239–4250. doi: 10.1093/nar/9.17.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley R. P., Mariano T. M., Siekierka J., Mathews M. B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986 Feb 14;44(3):391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. The nucleotide sequence of a low molecular weight ribonucleic acid from cells infected with adenovirus 2. J Biol Chem. 1971 Nov 25;246(22):6991–7009. [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Location of sequences on the adenovirus genome coding for the 5.5S RNA. Cell. 1975 Sep;6(1):1–4. doi: 10.1016/0092-8674(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railey J. F., 2nd, Wu G. J. Organization of multiple regulatory elements in the control region of the adenovirus type 2-specific VARNA1 gene: fine mapping with linker-scanning mutants. Mol Cell Biol. 1988 Mar;8(3):1147–1159. doi: 10.1128/mcb.8.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Forget B. G., Weissman S. M. RNA of low molecular weight in KB cells infected with adenovirus type 2. J Mol Biol. 1966 Jun;17(2):428–439. doi: 10.1016/s0022-2836(66)80153-5. [DOI] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Shenk T. Transcriptional control regions: nucleotide sequence requirements for initiation by RNA polymerase II and III. Curr Top Microbiol Immunol. 1981;93:25–46. doi: 10.1007/978-3-642-68123-3_3. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Hong J. S., Wei Y. F., Engler J. A. Nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 12. Gene. 1986;46(2-3):187–195. doi: 10.1016/0378-1119(86)90403-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snouwaert J., Bunick D., Hutchison C., Fowlkes D. M. Large numbers of random point and cluster mutations within the adenovirus VA I gene allow characterization of sequences required for efficient transcription. Nucleic Acids Res. 1987 Oct 26;15(20):8293–8303. doi: 10.1093/nar/15.20.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI mediates a translational stimulation which is not restricted to the viral mRNAs. EMBO J. 1985 Apr;4(4):957–964. doi: 10.1002/j.1460-2075.1985.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI: a positive regulator of mRNA translation. Mol Cell Biol. 1984 Apr;4(4):736–742. doi: 10.1128/mcb.4.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Defective RNA splicing in the absence of adenovirus-associated RNAI. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4690–4694. doi: 10.1073/pnas.83.13.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Gingeras T. R., Bullock P., Wong G., Gelinas R. E. Determination and analysis of adenovirus-2 DNA sequences which may include signals for late messenger RNA processing. J Mol Biol. 1979 Dec 5;135(2):413–433. doi: 10.1016/0022-2836(79)90444-3. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]