Abstract
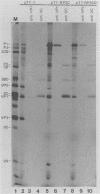
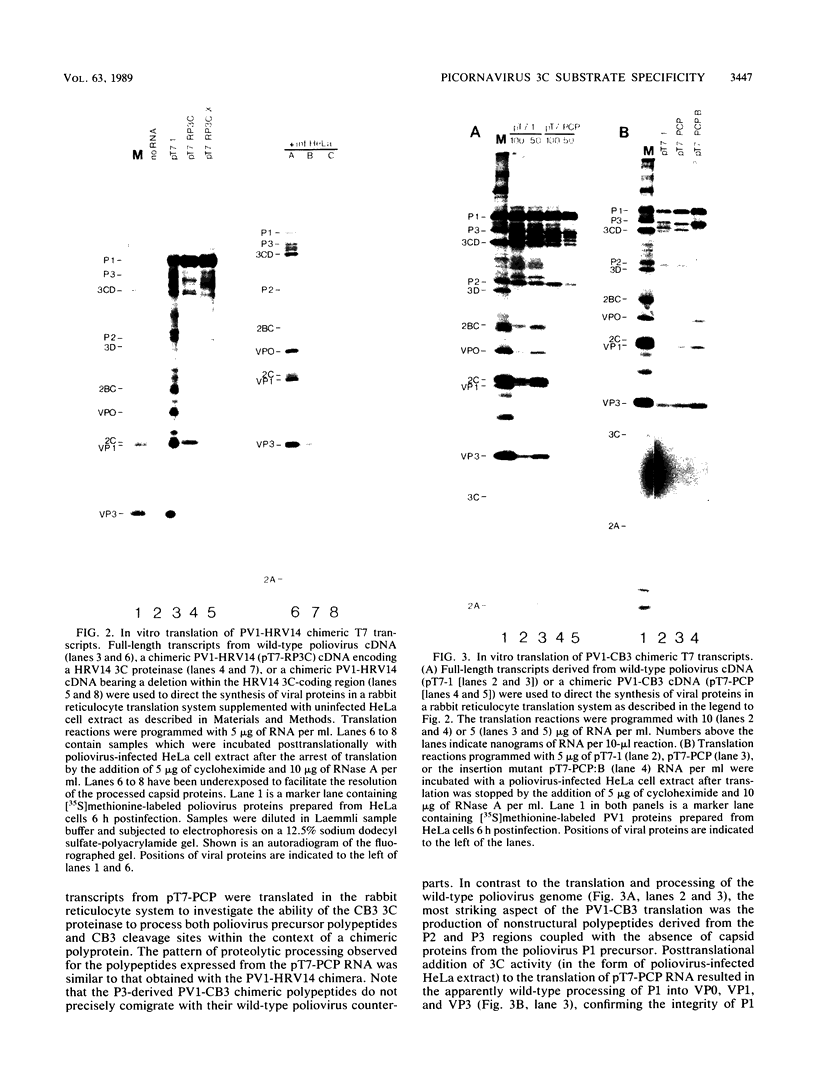
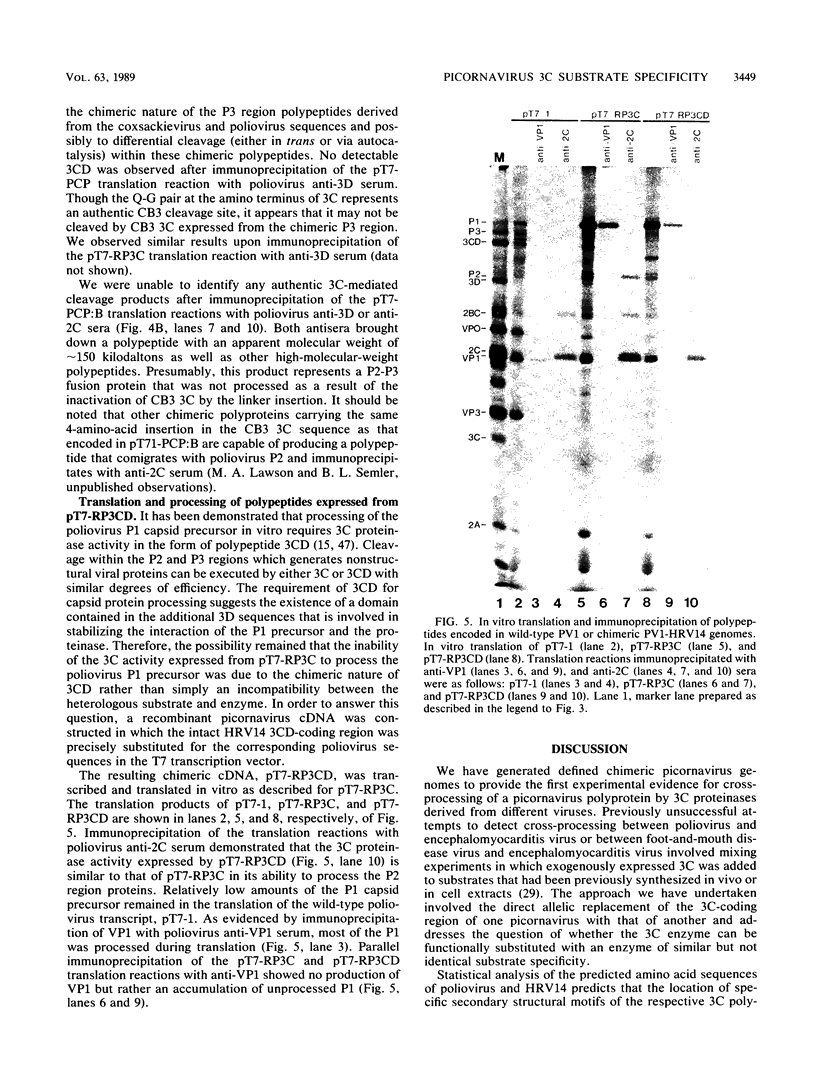
Cross-species proteolytic processing was demonstrated by the 3C proteinases of human rhinovirus 14 and coxsackievirus B3 on poliovirus-specific polypeptide precursors. Chimeric picornavirus cDNA genomes were constructed in a T7 transcription vector in which the poliovirus 3C coding region was substituted with the corresponding allele from human rhinovirus 14 or coxsackievirus B3. In vitro translation and processing of the polypeptides encoded by the chimeric genomes demonstrated that the proteolytic processing of poliovirus P2 region (nonstructural) proteins could be functionally substituted by the heterologous proteinases. In contrast, the 3C proteinase activities expressed from the chimeric genomes were incapable of recognizing the poliovirus-specific processing sites within the capsid precursor. Since the amino acid sequences flanking and inclusive of the P2 region cleavage sites of the three viruses are not stringently conserved, these results provide evidence for the existence of common conformational determinants necessary for 3C-mediated processing.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979 Sep;97(2):396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Burke K. L., Dunn G., Ferguson M., Minor P. D., Almond J. W. Antigen chimaeras of poliovirus as potential new vaccines. Nature. 1988 Mar 3;332(6159):81–82. doi: 10.1038/332081a0. [DOI] [PubMed] [Google Scholar]
- Callahan P. L., Mizutani S., Colonno R. J. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc Natl Acad Sci U S A. 1985 Feb;82(3):732–736. doi: 10.1073/pnas.82.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalt P. G., Semler B. L. Site-directed mutagenesis of proteinase 3C results in a poliovirus deficient in synthesis of viral RNA polymerase. J Virol. 1987 Jul;61(7):2162–2170. doi: 10.1128/jvi.61.7.2162-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Semler B. L., Jackson R. J., Hanecak R., Duprey E., Wimmer E. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J Virol. 1984 May;50(2):507–514. doi: 10.1128/jvi.50.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Schleif W. A., Colonno R. J., Wimmer E. Antigenic conservation and divergence between the viral-specific proteins of poliovirus type 1 and various picornaviruses. Virology. 1985 Jan 15;140(1):13–20. doi: 10.1016/0042-6822(85)90441-6. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Blinov V. M., Donchenko A. P. Poliovirus-encoded proteinase 3C: a possible evolutionary link between cellular serine and cysteine proteinase families. FEBS Lett. 1986 Jan 6;194(2):253–257. doi: 10.1016/0014-5793(86)80095-3. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Ariga H., Anderson C. W., Wimmer E. Expression of a cloned gene segment of poliovirus in E. coli: evidence for autocatalytic production of the viral proteinase. Cell. 1984 Jul;37(3):1063–1073. doi: 10.1016/0092-8674(84)90441-0. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Towatari T., Ray J., Korant B. D., Petteway S. R., Jr Expression and site-specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. H., Semler B. L. Defined recombinants of poliovirus and coxsackievirus: sequence-specific deletions and functional substitutions in the 5'-noncoding regions of viral RNAs. Virology. 1988 Jan;162(1):47–57. doi: 10.1016/0042-6822(88)90393-5. [DOI] [PubMed] [Google Scholar]
- Jore J., De Geus B., Jackson R. J., Pouwels P. H., Enger-Valk B. E. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1 in vitro. J Gen Virol. 1988 Jul;69(Pt 7):1627–1636. doi: 10.1099/0022-1317-69-7-1627. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kohara M., Abe S., Komatsu T., Tago K., Arita M., Nomoto A. A recombinant virus between the Sabin 1 and Sabin 3 vaccine strains of poliovirus as a possible candidate for a new type 3 poliovirus live vaccine strain. J Virol. 1988 Aug;62(8):2828–2835. doi: 10.1128/jvi.62.8.2828-2835.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Brzin J., Turk V. Cystatin, a protein inhibitor of cysteine proteases alters viral protein cleavages in infected human cells. Biochem Biophys Res Commun. 1985 Mar 29;127(3):1072–1076. doi: 10.1016/s0006-291x(85)80054-1. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- La Monica N., Almond J. W., Racaniello V. R. A mouse model for poliovirus neurovirulence identifies mutations that attenuate the virus for humans. J Virol. 1987 Sep;61(9):2917–2920. doi: 10.1128/jvi.61.9.2917-2920.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Kuhn R. J., Arita M., Kawamura N., Nomoto A., Wimmer E. Poliovirus type 1/type 3 antigenic hybrid virus constructed in vitro elicits type 1 and type 3 neutralizing antibodies in rabbits and monkeys. Proc Natl Acad Sci U S A. 1988 May;85(9):3203–3207. doi: 10.1073/pnas.85.9.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Harris K. S., Pallai P. V., Wimmer E. Poliovirus proteinase 3C: large-scale expression, purification, and specific cleavage activity on natural and synthetic substrates in vitro. J Virol. 1988 Dec;62(12):4586–4593. doi: 10.1128/jvi.62.12.4586-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Semler B. L., Omilianowski D. R., Anderson C. W., Wimmer E., Rueckert R. R. Protein processing map of poliovirus. J Virol. 1984 Mar;49(3):873–880. doi: 10.1128/jvi.49.3.873-880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. Picornaviral processing: some new ideas. J Cell Biochem. 1987 Mar;33(3):191–198. doi: 10.1002/jcb.240330306. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Emmert A. Modulation of the expression of poliovirus proteins in reticulocyte lysates. Virology. 1986 Jan 30;148(2):255–267. doi: 10.1016/0042-6822(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Dorner L. F., Anderson C. W., Wimmer E. Poliovirus RNA synthesis in vitro: structural elements and antibody inhibition. Virology. 1983 Apr 30;126(2):624–635. doi: 10.1016/s0042-6822(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Johnson V. H., Dewalt P. G., Ypma-Wong M. F. Site-specific mutagenesis of cDNA clones expressing a poliovirus proteinase. J Cell Biochem. 1987 Jan;33(1):39–51. doi: 10.1002/jcb.240330105. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Johnson V. H., Tracy S. A chimeric plasmid from cDNA clones of poliovirus and coxsackievirus produces a recombinant virus that is temperature-sensitive. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1777–1781. doi: 10.1073/pnas.83.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Minor P. D., Almond J. W. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., Liu H. L. Molecular cloning and partial characterization of the coxsackievirus B3 genome. Brief report. Arch Virol. 1985;85(1-2):157–163. doi: 10.1007/BF01317016. [DOI] [PubMed] [Google Scholar]
- Werner G., Rosenwirth B., Bauer E., Seifert J. M., Werner F. J., Besemer J. Molecular cloning and sequence determination of the genomic regions encoding protease and genome-linked protein of three picornaviruses. J Virol. 1986 Mar;57(3):1084–1093. doi: 10.1128/jvi.57.3.1084-1093.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Filman D. J., Hogle J. M., Semler B. L. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at Gln-Gly pairs. J Biol Chem. 1988 Nov 25;263(33):17846–17856. [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. Processing determinants required for in vitro cleavage of the poliovirus P1 precursor to capsid proteins. J Virol. 1987 Oct;61(10):3181–3189. doi: 10.1128/jvi.61.10.3181-3189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]