Abstract
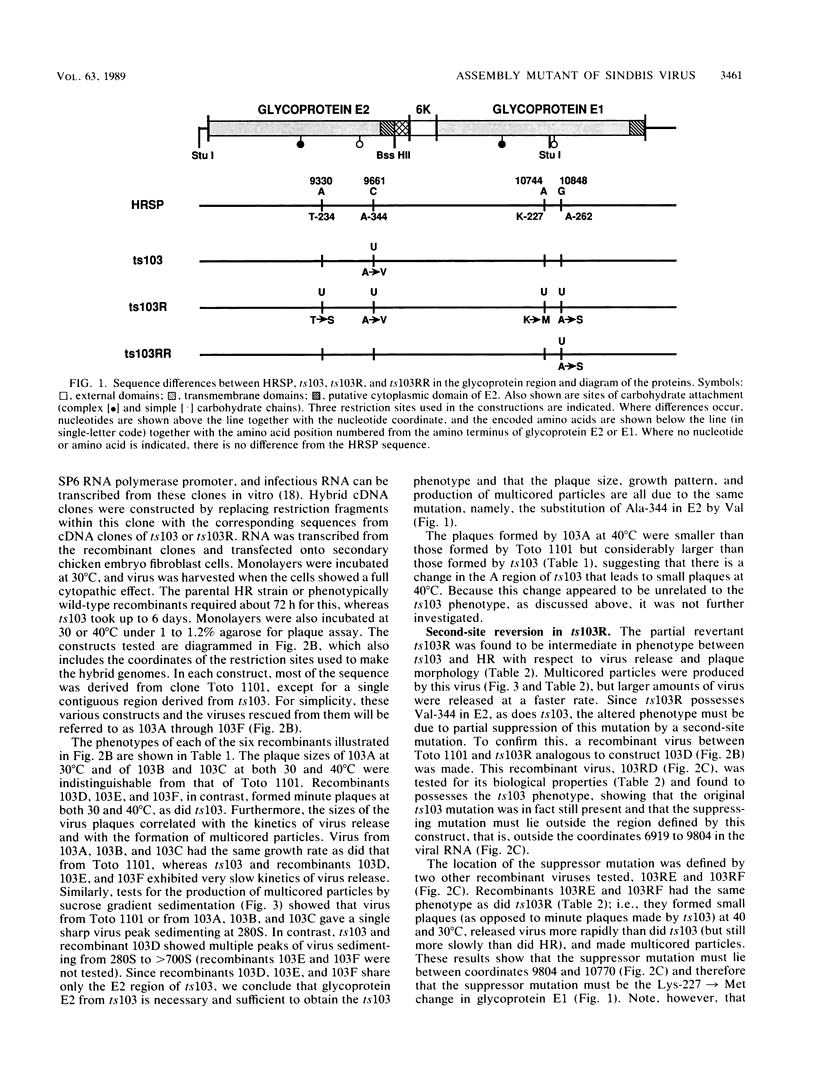
Sindbis virus mutant ts103 is aberrant in the assembly of virus particles. During virus budding, proper nucleocapsid-glycoprotein interactions fail to occur such that particles containing many nucleocapsids are formed, and the final yield of virus is low. We have determined that a mutation in the external domain of glycoprotein E2, Ala-344----Val, is the change that leads to this phenotype. Mapping was done by making recombinant viruses between ts103 and a parental strain of the virus, using a full-length cDNA clone of Sindbis virus from which infectious RNA can be transcribed, together with sequence analysis of the region of the genome shown in this way to contain the ts103 lesion. A partial revertant of ts103, called ts103R, was also mapped and sequenced and found to be a second-site revertant in which a change in glycoprotein E1 from lysine to methionine at position 227 partially suppresses the phenotypic effects of the change at E2 position 344. An analysis of revertants from ts103 mutants in which the Ala----Val change had been transferred into a defined background showed that pseudorevertants were more likely to arise than were true revertants and that the ts103 change itself reverted very infrequently. The assembly defect in ts103 appeared to result from weakened interactions between the virus membrane glycoproteins or between these glycoproteins and the nucleocapsid during budding. Both the E2 mutation leading to the defect in virus assembly and the suppressor mutation in glycoprotein E1 are in the domains external to the lipid bilayer and thus in domains that cannot interact directly with the nucleocapsid. This suggests that in ts103, either the E1-E2 heterodimers or the trimeric spikes (consisting of three E1-E2 heterodimers) are unstable or have an aberrant configuration, and thus do not interact properly with the nucleocapsid, or cannot assembly correctly to form the proper icosahedral array on the surface of the virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C., Bell J. R., Lenches E. M., Strauss E. G., Strauss J. H. Sequence analysis of two mutants of Sindbis virus defective in the intracellular transport of their glycoproteins. J Mol Biol. 1983 Jul 25;168(1):87–102. doi: 10.1016/s0022-2836(83)80324-6. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Brown B., Brown D. T. Evidence for a change in capsid morphology during Sindbis virus envelopment. Virus Res. 1984;1(4):297–302. doi: 10.1016/0168-1702(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Durbin R. K., Stollar V. Sequence analysis of the E2 gene of a hyperglycosylated, host restricted mutant of Sindbis virus and estimation of mutation rate from frequency of revertants. Virology. 1986 Oct 15;154(1):135–143. doi: 10.1016/0042-6822(86)90436-8. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., DiSalvo J., Rice C. M., Strauss J. H., Strauss E. G. Sindbis virus mutant ts20 of complementation group E contains a lesion in glycoprotein E2. Virology. 1986 May;151(1):10–20. doi: 10.1016/0042-6822(86)90099-1. [DOI] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988 Jul;62(7):2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. S., Strauss E. G., Strauss J. H. Effect of ionic strength on the binding of Sindbis virus to chick cells. J Virol. 1974 May;13(5):1030–1036. doi: 10.1128/jvi.13.5.1030-1036.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Synthesis, cleavage and sequence analysis of DNA complementary to the 26 S messenger RNA of Sindbis virus. J Mol Biol. 1981 Aug 15;150(3):315–340. doi: 10.1016/0022-2836(81)90550-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Birdwell C. R., Lenches E. M., Staples S. E., Strauss J. H. Mutants of Sindbis virus. II. Characterization of a maturation-defective mutant, ts103. Virology. 1977 Oct 1;82(1):122–149. doi: 10.1016/0042-6822(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Stamreich-Martin M. A. Growth and release of several alphaviruses in chick and BHK cells. J Gen Virol. 1980 Aug;49(2):297–307. doi: 10.1099/0022-1317-49-2-297. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Strauss E. G. Mutants of Sindbis virus. III. Host polypeptides present in purified HR and ts103 virus particles. J Virol. 1978 Nov;28(2):466–474. doi: 10.1128/jvi.28.2.466-474.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Inhibition of Sindbis virus production by media of low ionic strength: intracellular events and requirements for reversal. J Virol. 1970 Jan;5(1):60–71. doi: 10.1128/jvi.5.1.60-71.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]


